Validation of volatile metabolites of pulmonary oxidative injury: a bench to bedside study
- PMID: 36949963
- PMCID: PMC10026006
- DOI: 10.1183/23120541.00427-2022
Validation of volatile metabolites of pulmonary oxidative injury: a bench to bedside study
Abstract
Background: Changes in exhaled volatile organic compounds (VOCs) can be used to discriminate between respiratory diseases, and increased concentrations of hydrocarbons are commonly linked to oxidative stress. However, the VOCs identified are inconsistent between studies, and translational studies are lacking.
Methods: In this bench to bedside study, we captured VOCs in the headspace of A549 epithelial cells after exposure to hydrogen peroxide (H2O2), to induce oxidative stress, using high-capacity polydimethylsiloxane sorbent fibres. Exposed and unexposed cells were compared using targeted and untargeted analysis. Breath samples of invasively ventilated intensive care unit patients (n=489) were collected on sorbent tubes and associated with the inspiratory oxygen fraction (F IO2 ) to reflect pulmonary oxidative stress. Headspace samples and breath samples were analysed using gas chromatography and mass spectrometry.
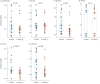
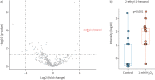
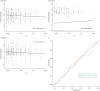
Results: In the cell, headspace octane concentration was decreased after oxidative stress (p=0.0013), while the other VOCs were not affected. 2-ethyl-1-hexanol showed an increased concentration in the headspace of cells undergoing oxidative stress in untargeted analysis (p=0.00014). None of the VOCs that were linked to oxidative stress showed a significant correlation with F IO2 (Rs range: -0.015 to -0.065) or discriminated between patients with F IO2 ≥0.6 or below (area under the curve range: 0.48 to 0.55).
Conclusion: Despite a comprehensive translational approach, validation of known and novel volatile biomarkers of oxidative stress was not possible in patients at risk of pulmonary oxidative injury. The inconsistencies observed highlight the difficulties faced in VOC biomarker validation, and that caution is warranted in the interpretation of the pathophysiological origin of discovered exhaled breath biomarkers.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: D. Fenn has nothing to disclose. Conflict of interest: T.A. Lilien has nothing to disclose. Conflict of interest: L.A. Hagens has nothing to disclose. Conflict of interest: M.R. Smit has nothing to disclose. Conflict of interest: N.F.L. Heijnen has nothing to disclose. Conflict of interest: A.M. Tuip-de Boer has nothing to disclose. Conflict of interest: A.H. Neerincx has nothing to disclose. Conflict of interest: K. Golebski has nothing to disclose. Conflict of interest: D.C.J.J. Bergmans has nothing to disclose. Conflict of interest: R.M. Schnabel has nothing to disclose. Conflict of interest: M.J. Schultz has nothing to disclose. Conflict of interest: A.H. Maitland-van der Zee has received research grants outside the submitted work from GSK, Boehringer Ingelheim and Vertex; she is the principal investigator of a P4O2 (Precision Medicine for more Oxygen) public–private partnership sponsored by Health Holland involving many private partners that contribute in cash and/or in kind (Boehringer Ingelheim, Breathomix, Fluidda, Ortec Logiqcare, Philips, Quantib-U, Roche, Smartfish, SODAQ, Thirona, TopMD and Novartis); and she has served in advisory boards for AstraZeneca, GSK and Boehringer Ingelheim, with money paid to her institution. Conflict of interest: P. Brinkman has nothing to disclose. Conflict of interest: L.D.J. Bos reports grants from the Dutch Lung Foundation (Young investigator grant), grants from the Dutch Lung Foundation and Health Holland (Public–Private Partnership grant), grants from the Dutch Lung Foundation (Dirkje Postma Award), grants from IMI COVID19 initiative, and grants from Amsterdam UMC fellowship, outside the submitted work; he has also served in advisory capacity for Santhera and Janssen with money paid to his institution.
Figures

References
LinkOut - more resources
Full Text Sources
