Diagnostic Efficacy of RealStar SARS-CoV-2 Reverse Transcription-Polymerase Chain Reaction (RT-PCR) in Comparison to GeneXpert System for the Detection of COVID-19
- PMID: 36949993
- PMCID: PMC10028309
- DOI: 10.7759/cureus.35158
Diagnostic Efficacy of RealStar SARS-CoV-2 Reverse Transcription-Polymerase Chain Reaction (RT-PCR) in Comparison to GeneXpert System for the Detection of COVID-19
Abstract
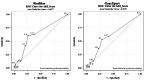
Background and objective The coronavirus disease 2019 (COVID-19) pandemic has become a major health concern due to the rapid transmission of the virus that causes it: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To address the growing demand on healthcare systems to control this pandemic, more effective diagnostic methods need to be applied. In this study, we aimed to compare the efficacy of RealStar® SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) versus the GeneXpert® system. Methods A retrospective cross-sectional study was conducted in the central lab of King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. Data from all nasopharyngeal swabs (NPS) (150,000) submitted for SARS-CoV-2 analysis from July 2020 to July 2021 were reviewed retrospectively. Furthermore, all NPS (n=384) that were analyzed on both the RealStar® SARS-CoV-2 RT-PCR and GeneXpert® systems for confirmatory purposes were included in the study. Acute respiratory illness (ARI) screening forms of the selected samples were reviewed from the electronic database (BestCare system), and they were analyzed and compared at one point in time; therefore, a cross-sectional study was found to be the best suitable study design. Using the statistical analysis software, the receiver operating characteristic (ROC) curve was obtained to compare the sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV). The test was considered significant if the area under the curve (AUC) value was >0.5. Results The diagnostic performance of the RealStar® and GeneXpert® assays in detecting SARS-CoV-2 was evaluated using ROC curve analysis, which showed AUCs of 0.597 and 0.637, respectively. In addition, 35% of the total results fell into a substantial agreement of 0.76 (95% CI: 0.6626-0.8732). The majority of the NPS were reported negative by both RealStar® (246, 80.66%) and GeneXpert® (226, 74.10%). Most samples (210, 68.85%) were obtained from asymptomatic patients, scoring less than 4 (ARI <4) based on the ARI screening form. Conclusion Based on the AUC of ROC, there is no significant difference in the performance characteristics between the RealStar® RT-PCR and GeneXpert® in detecting COVID-19.
Keywords: acute respiratory illness (ari) score; covid-19; genexpert®; realstar® sars-cov-2 rt–pcr; saudi arabia.
Copyright © 2023, Jawdat et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- MOH: Coronavirus disease 2019 guidelines. [ Jan; 2020 ]. 2020. https://www.moh.gov.sa/CCC/healthp/regulations/Documents/Coronavirus%20D... https://www.moh.gov.sa/CCC/healthp/regulations/Documents/Coronavirus%20D...
-
- Payne S. Viruses. Vol. 1. Amsterdam, Netherlands: Elsevier; 2017. Family Coronaviridae; pp. 149–158.
LinkOut - more resources
Full Text Sources
Miscellaneous