Evaluation of therapeutic effects of tetramethylpyrazine nitrone in Alzheimer's disease mouse model and proteomics analysis
- PMID: 36950017
- PMCID: PMC10025301
- DOI: 10.3389/fphar.2023.1082602
Evaluation of therapeutic effects of tetramethylpyrazine nitrone in Alzheimer's disease mouse model and proteomics analysis
Abstract
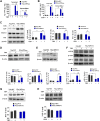
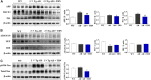
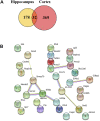
The pathophysiology of Alzheimer's disease (AD) is multifactorial with characteristic extracellular accumulation of amyloid-beta (Aβ) and intraneuronal aggregation of hyperphosphorylated tau in the brain. Development of disease-modifying treatment for AD has been challenging. Recent studies suggest that deleterious alterations in neurovascular cells happens in parallel with Aβ accumulation, inducing tau pathology and necroptosis. Therefore, therapies targeting cellular Aβ and tau pathologies may provide a more effective strategy of disease intervention. Tetramethylpyrazine nitrone (TBN) is a nitrone derivative of tetramethylpyrazine, an active ingredient from Ligusticum wallichii Franchat (Chuanxiong). We previously showed that TBN is a potent scavenger of free radicals with multi-targeted neuroprotective effects in rat and monkey models of ischemic stroke. The present study aimed to investigate the anti-AD properties of TBN. We employed AD-related cellular model (N2a/APPswe) and transgenic mouse model (3×Tg-AD mouse) for mechanistic and behavioral studies. Our results showed that TBN markedly improved cognitive functions and reduced Aβ and hyperphosphorylated tau levels in mouse model. Further investigation of the underlying mechanisms revealed that TBN promoted non-amyloidogenic processing pathway of amyloid precursor protein (APP) in N2a/APPswe in vitro. Moreover, TBN preserved synapses from dendritic spine loss and upregulated synaptic protein expressions in 3×Tg-AD mice. Proteomic analysis of 3×Tg-AD mouse hippocampal and cortical tissues showed that TBN induced neuroprotective effects through modulating mitophagy, MAPK and mTOR pathways. In particular, TBN significantly upregulated PINK1, a key protein for mitochondrial homeostasis, implicating PINK1 as a potential therapeutic target for AD. In summary, TBN improved cognitive functions in AD-related mouse model, inhibited Aβ production and tau hyperphosphorylation, and rescued synaptic loss and neuronal damage. Multiple mechanisms underlie the anti-AD effects of TBN including the modulation of APP processing, mTOR signaling and PINK1-related mitophagy.
Keywords: PINK1; alhzheimer disease; amyloid beta; proteomic analysis; tetramethylpyrazine nitrone.
Copyright © 2023 Zhou, Huang, Wang, Zhang, Liu, Hou, Yang and Hoi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











Similar articles
-
Tetramethylpyrazine Nitrone (TBN) Reduces Amyloid β Deposition in Alzheimer's Disease Models by Modulating APP Expression, BACE1 Activity, and Autophagy Pathways.Pharmaceuticals (Basel). 2024 Jul 30;17(8):1005. doi: 10.3390/ph17081005. Pharmaceuticals (Basel). 2024. PMID: 39204110 Free PMC article.
-
Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer's disease.Theranostics. 2020 Sep 26;10(25):11794-11819. doi: 10.7150/thno.44152. eCollection 2020. Theranostics. 2020. PMID: 33052247 Free PMC article.
-
Hippocampal Proteomic Alteration in Triple Transgenic Mouse Model of Alzheimer's Disease and Implication of PINK 1 Regulation in Donepezil Treatment.J Proteome Res. 2019 Apr 5;18(4):1542-1552. doi: 10.1021/acs.jproteome.8b00818. Epub 2018 Dec 7. J Proteome Res. 2019. PMID: 30484658
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
-
Therapeutic Strategies Targeting Amyloid-β in Alzheimer's Disease.Curr Alzheimer Res. 2019;16(5):418-452. doi: 10.2174/1567205016666190321163438. Curr Alzheimer Res. 2019. PMID: 30907320 Review.
Cited by
-
The crosstalk between mitochondrial quality control and metal-dependent cell death.Cell Death Dis. 2024 Apr 27;15(4):299. doi: 10.1038/s41419-024-06691-w. Cell Death Dis. 2024. PMID: 38678018 Free PMC article. Review.
-
Tetramethylpyrazine Nitrone (TBN) Reduces Amyloid β Deposition in Alzheimer's Disease Models by Modulating APP Expression, BACE1 Activity, and Autophagy Pathways.Pharmaceuticals (Basel). 2024 Jul 30;17(8):1005. doi: 10.3390/ph17081005. Pharmaceuticals (Basel). 2024. PMID: 39204110 Free PMC article.
-
Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke.Antioxidants (Basel). 2023 Dec 12;12(12):2102. doi: 10.3390/antiox12122102. Antioxidants (Basel). 2023. PMID: 38136221 Free PMC article. Review.
-
Tetramethylpyrazine inhibits ferroptosis in spinal cord injury by regulating iron metabolism through the NRF2/ARE pathway.Front Pharmacol. 2024 Nov 15;15:1503064. doi: 10.3389/fphar.2024.1503064. eCollection 2024. Front Pharmacol. 2024. PMID: 39619616 Free PMC article.
References
-
- Ali A. A. (2016). Alzheimer's disease: Pathophysiology, hypotheses and treatment strategies. Acta Psychopathol. 2. 10.4172/2469-6676.100049 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

