Metabolomic and cultivation insights into the tolerance of the spacecraft-associated Acinetobacter toward Kleenol 30, a cleanroom floor detergent
- PMID: 36950167
- PMCID: PMC10025500
- DOI: 10.3389/fmicb.2023.1090740
Metabolomic and cultivation insights into the tolerance of the spacecraft-associated Acinetobacter toward Kleenol 30, a cleanroom floor detergent
Abstract
Introduction: Stringent cleaning procedures during spacecraft assembly are critical to maintaining the integrity of life-detection missions. To ensure cleanliness, NASA spacecraft are assembled in cleanroom facilities, where floors are routinely cleansed with Kleenol 30 (K30), an alkaline detergent.
Methods: Through metabolomic and cultivation approaches, we show that cultures of spacecraft-associated Acinetobacter tolerate up to 1% v/v K30 and are fully inhibited at ≥2%; in comparison, NASA cleanrooms are cleansed with ~0.8-1.6% K30.
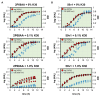
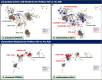
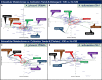
Results: For A. johnsonii 2P08AA (isolated from a cleanroom floor), cultivations with 0.1% v/v K30 yield (1) no changes in cell density at late-log phase, (2) modest decreases in growth rate (~17%), (3) negligible lag phase times, (4) limited changes in the intracellular metabolome, and (5) increases in extracellular sugar acids, monosaccharides, organic acids, and fatty acids. For A. radioresistens 50v1 (isolated from a spacecraft surface), cultivations yield (1) ~50% survivals, (2) no changes in growth rate, (3) ~70% decreases in the lag phase time, (4) differential changes in intracellular amino acids, compatible solutes, nucleotide-related metabolites, dicarboxylic acids, and saturated fatty acids, and (5) substantial yet differential impacts to extracellular sugar acids, monosaccharides, and organic acids.
Discussion: These combined results suggest that (1) K30 manifests strain-dependent impacts on the intracellular metabolomes, cultivation kinetics, and survivals, (2) K30 influences extracellular trace element acquisition in both strains, and (3) K30 is better tolerated by the floor-associated strain. Hence, this work lends support towards the hypothesis that repeated cleansing during spacecraft assembly serve as selective pressures that promote tolerances towards the cleaning conditions.
Keywords: Acinetobacter; cleanrooms; detergent; metabolomics; planetary protection; spacecraft; survival.
Copyright © 2023 Mogul, Miller, Ramos and Lalla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agalloco J. P., Carleton F. J. (2007). Validation of Pharmaceutical Processes. Boca Raton, Florida: CRC Press.
-
- Angyal S. (1973). “Complex formation between sugars and metal ions” in Carbohydrate Chemistry–VI. ed. Doane W. M., Northern Regional Research Laboratory, Peoria, Illinois (Amsterdam, Netherlands: Elsevier; ), 131–146.
-
- Barupal D. K., Haldiya P. K., Wohlgemuth G., Kind T., Kothari S. L., Pinkerton K. E., et al. (2012). MetaMapp: mapping and visualizing metabolomic data by integrating information from biochemical pathways and chemical and mass spectral similarity. BMC Bioinform. 13, 1–15. doi: 10.1186/1471-2105-13-99 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

