Direct GDP-KRASG12C inhibitors and mechanisms of resistance: the tip of the iceberg
- PMID: 36950276
- PMCID: PMC10026147
- DOI: 10.1177/17588359231160141
Direct GDP-KRASG12C inhibitors and mechanisms of resistance: the tip of the iceberg
Abstract
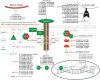
Kirsten rat sarcoma viral oncogene homolog mutations are observed in 25% of lung adenocarcinoma and 40% of these are G12C mutations. Historically, no approved targeted agents were available for patients with any KRAS mutation, and response rates to standard-of-care therapies were suboptimal. Newly developed inhibitors directed toward KRASG12C have been successful in clinical trials with overall response rates ranging between 32% and 46%, and two FDA approvals were granted in May 2021 and December 2022 as second-line or later monotherapies. However, rapid tumor resistance complicates their use as a monotherapy. With the rapid development of this novel class of inhibitors, it is important to discern the different types of tumor resistance that may arise and how each can differently contribute to tumor growth and survival. G12C inhibitor resistance is under investigation and combinations of therapies with G12C inhibitors have been proposed. Much of this insight is gleaned from preclinical investigations, as our knowledge of clinical resistance is in its infancy. In this review, we summarize the preclinical development of KRASG12C inhibitors, their clinical evaluations, different types of resistance mechanisms to these compounds, and ways of overcoming them. Finally, we underscore the importance of basic and translational investigations of these molecules in a landscape where their clinical evaluations garner the most attention, and we set the stage for what is to come.
Keywords: G12C; KRAS; clinical trial; drug resistance; preclinical; targeted therapy.
© The Author(s), 2023.
Conflict of interest statement
Adrian Sacher – Research funding or support: AstraZeneca, Genentech, BMS, Guardant. Institutional Sponsored Research Funding: AstraZeneca, Genentech, Amgen, Merck, GSK, Spectrum, Pfizer, Lilly, RevMed, Iovance, CRISPR Therapeutics Ming-Sound Tsao – Consultancy honoraria: Abbvie, Amgen, AstraZeneca, Bayer, BMS, Daiichi-Sankyo, Lilly, Regeneron, Sanofi, Nucleix. Research grant: Bayer, AstraZeneca, Sanofi
Figures


References
-
- Amgen. LUMAKRAS® (SOTORASIB) Receives approval in japan for patients with Kras G12c-mutated advanced non-small cell lung cancer, https://www.amgen.com/newsroom/press-releases/2022/01/lumakras-sotorasib... (2022, accessed 20 January 2022).
-
- Li B, Skoulidis F, Falchook G, et al.. PS01.07 registrational phase 2 trial of sotorasib in KRAS p.G12C mutant NSCLC: first disclosure of the codebreak 100 primary analysis. J Thorac Oncol 2021; 16: S61.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

