Protein interaction studies in human induced neurons indicate convergent biology underlying autism spectrum disorders
- PMID: 36950384
- PMCID: PMC10025425
- DOI: 10.1016/j.xgen.2022.100250
Protein interaction studies in human induced neurons indicate convergent biology underlying autism spectrum disorders
Abstract
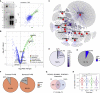
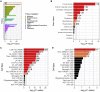
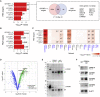
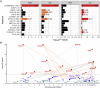
Autism spectrum disorders (ASDs) have been linked to genes with enriched expression in the brain, but it is unclear how these genes converge into cell-type-specific networks. We built a protein-protein interaction network for 13 ASD-associated genes in human excitatory neurons derived from induced pluripotent stem cells (iPSCs). The network contains newly reported interactions and is enriched for genetic and transcriptional perturbations observed in individuals with ASDs. We leveraged the network data to show that the ASD-linked brain-specific isoform of ANK2 is important for its interactions with synaptic proteins and to characterize a PTEN-AKAP8L interaction that influences neuronal growth. The IGF2BP1-3 complex emerged as a convergent point in the network that may regulate a transcriptional circuit of ASD-associated genes. Our findings showcase cell-type-specific interactomes as a framework to complement genetic and transcriptomic data and illustrate how both individual and convergent interactions can lead to biological insights into ASDs.
Keywords: IP-MS; autism spectrum disorders; exome sequencing; induced excitatory neurons; protein-protein interactions.
© 2023 The Authors.
Conflict of interest statement
K.C.E. is a co-founder of Q-State Biosciences, Quralis, and Enclear and is currently employed at BioMarin Pharmaceutical.
Figures




References
-
- Weiner D.J., Wigdor E.M., Ripke S., Walters R.K., Kosmicki J.A., Grove J., Samocha K.E., Goldstein J.I., Okbay A., Bybjerg-Grauholm J., et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat. Genet. 2017;49:978–985. doi: 10.1038/ng.3863. - DOI - PMC - PubMed
-
- Satterstrom F.K., Kosmicki J.A., Wang J., Breen M.S., De Rubeis S., An J.Y., Peng M., Collins R., Grove J., Klei L., et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–584.e23. doi: 10.1016/j.cell.2019.12.036. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

