Effectiveness of zebrafish models in understanding human diseases-A review of models
- PMID: 36950605
- PMCID: PMC10025926
- DOI: 10.1016/j.heliyon.2023.e14557
Effectiveness of zebrafish models in understanding human diseases-A review of models
Abstract
Understanding the detailed mechanism behind every human disease, disorder, defect, and deficiency is a daunting task concerning the clinical diagnostic tools for patients. Hence, a closely resembling living or simulated model is of paramount interest for the development and testing of a probable novel drug for rectifying the conditions pertaining to the various ailments. The animal model that can be easily genetically manipulated to suit the study of the therapeutic motive is an indispensable asset and within the last few decades, the zebrafish models have proven their effectiveness by becoming such potent human disease models with their use being extended to various avenues of research to understand the underlying mechanisms of the diseases. As zebrafish are explored as model animals in understanding the molecular basis and genetics of many diseases owing to the 70% genetic homology between the human and zebrafish genes; new and fascinating facts about the diseases are being surfaced, establishing it as a very powerful tool for upcoming research. These prospective research areas can be explored in the near future using zebrafish as a model. In this review, the effectiveness of the zebrafish as an animal model against several human diseases such as osteoporosis, atrial fibrillation, Noonan syndrome, leukemia, autism spectrum disorders, etc. has been discussed.
Keywords: Experimental disease models; Human disease models; Teleost disease model; Zebrafish model; Zebrafish translational research.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

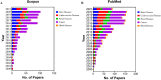


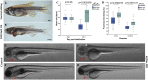

References
-
- Elson C.O., Balfour Sartor R., Tennyson G.S., Riddell R.H., Ii SPECIAL reports and reviews experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. - PubMed
-
- Zizioli D., Mione M., Varinelli M., Malagola M., Bernardi S., Alghisi E., Borsani G., Finazzi D., Monti E., Presta M., Russo D. Zebrafish disease models in hematology: highlights on biological and translational impact. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865:620–633. doi: 10.1016/j.bbadis.2018.12.015. - DOI - PubMed
-
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., McLaren S., Sealy I., Caccamo M., Churcher C., Scott C., Barrett J.C., Koch R., Rauch G.J., White S., Chow W., Kilian B., Quintais L.T., Guerra-Assunção J.A., Zhou Y., Gu Y., Yen J., Vogel J.H., Eyre T., Redmond S., Banerjee R., Chi J., Fu B., Langley E., Maguire S.F., Laird G.K., Lloyd D., Kenyon E., Donaldson S., Sehra H., Almeida-King J., Loveland J., Trevanion S., Jones M., Quail M., Willey D., Hunt A., Burton J., Sims S., McLay K., Plumb B., Davis J., Clee C., Oliver K., Clark R., Riddle C., Eliott D., Threadgold G., Harden G., Ware D., Mortimer B., Kerry G., Heath P., Phillimore B., Tracey A., Corby N., Dunn M., Johnson C., Wood J., Clark S., Pelan S., Griffiths G., Smith M., Glithero R., Howden P., Barker N., Stevens C., Harley J., Holt K., Panagiotidis G., Lovell J., Beasley H., Henderson C., Gordon D., Auger K., Wright D., Collins J., Raisen C., Dyer L., Leung K., Robertson L., Ambridge K., Leongamornlert D., McGuire S., Gilderthorp R., Griffiths C., Manthravadi D., Nichol S., Barker G., Whitehead S., Kay M., Brown J., Murnane C., Gray E., Humphries M., Sycamore N., Barker D., Saunders D., Wallis J., Babbage A., Hammond S., Mashreghi-Mohammadi M., Barr L., Martin S., Wray P., Ellington A., Matthews N., Ellwood M., Woodmansey R., Clark G., Cooper J., Tromans A., Grafham D., Skuce C., Pandian R., Andrews R., Harrison E., Kimberley A., Garnett J., Fosker N., Hall R., Garner P., Kelly D., Bird C., Palmer S., Gehring I., Berger A., Dooley C.M., Ersan-Ürün Z., Eser C., Geiger H., Geisler M., Karotki L., Kirn A., Konantz J., Konantz M., Oberländer M., Rudolph-Geiger S., Teucke M., Osoegawa K., Zhu B., Rapp A., Widaa S., Langford C., Yang F., Carter N.P., Harrow J., Ning Z., Herrero J., Searle S.M.J., Enright A., Geisler R., Plasterk R.H.A., Lee C., Westerfield M., De Jong P.J., Zon L.I., Postlethwait J.H., Nüsslein-Volhard C., Hubbard T.J.P., Crollius H.R., Rogers J., Stemple D.L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

