Danlou tablet inhibits high-glucose-induced cardiomyocyte apoptosis via the miR-34a-SIRT1 axis
- PMID: 36950610
- PMCID: PMC10025156
- DOI: 10.1016/j.heliyon.2023.e14479
Danlou tablet inhibits high-glucose-induced cardiomyocyte apoptosis via the miR-34a-SIRT1 axis
Abstract
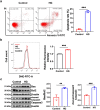
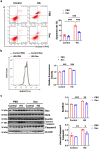
Diabetic cardiomyopathy (DCM) is highly prevalent and increases the risk of heart failure and sudden death. Therefore, proper and effective treatments for DCM are in urgent demand. Danlou tablet (Dan) is reported to confer protective effects on several heart diseases. However, to our knowledge, whether Dan provides protection against DCM is unclear. In this study, we explored the effect of Dan on DCM with the in vitro DCM model using AC16 cardiomyocytes. We found that Dan treatment significantly reduced cardiomyocyte apoptosis and oxidative stress in high-glucose (HG)-treated cardiomyocytes, as evidenced by decreased Annexin V-FITC+ cardiomyocytes, intracellular reactive oxygen species (ROS) levels, Bax/Bcl2 ratio, and cleaved-Caspase3/Caspase3 ratio. Interestingly, Dan treatment caused a decreased level of microRNA-34a (miR-34a), which could enhance cardiomyocyte apoptosis. Furthermore, miR-34a mimic blocked Dan's effect in apoptosis prevention. Finally, we observed that the miR-34a mimic effectively decreased the level of sirtuin 1 (SIRT1), while the miR-34a inhibitor increased the level of SIRT1. And downregulation of SIRT1 effectively reversed the effect of miR-34a inhibitor on cardiomyocyte apoptosis. Taken together, our study showed that Dan prevented HG-induced cardiomyocyte apoptosis through downregulating miR-34a and upregulating SIRT1. Our study has provided experimental support for the potential use of Dan in treating DCM. Further detailed study of Dan and the underlying mechanisms may shed light on the prevention and treatment of DCM.
Keywords: Cardiomyocyte apoptosis; Danlou tablet; Diabetic cardiomyopathy; SIRT1; miR-34a.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

