Ficus Benjamin's leaf, a native sorbent for the exclusion of Methyl violet 10B from aquatic media
- PMID: 36950624
- PMCID: PMC10025112
- DOI: 10.1016/j.heliyon.2023.e14295
Ficus Benjamin's leaf, a native sorbent for the exclusion of Methyl violet 10B from aquatic media
Abstract
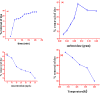
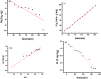
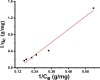
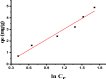
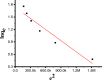
Due to the use of non-recyclable materials and the high costs of the technologies, removal of dyes from wastewater is becoming more and more pricey. This paper presents the capacity of Ficus Benjamina (FB) leaf powder to eradicate methyl violet dye 10 B (MV) in an aqueous fluid. The surface characteristics and presence of various functional groups on the surface of sorbent were revealed by SEM and FTIR studies. Diverse constraints on the elimination of methyl violet 10 B in an aqueous environment were also studied, including starting dye concentration, temperature, and contact duration. The Elovich & liquid film diffusion models, along with Lagergren first-order, pseudo-second-order, Bangham, and modified Freundlich modeling operated to assess kinetics. Experiments confirmed the pseudo-second-order concept. To investigate tentative data, multiple linear Langmuir, Freundlich, Temkin, as well as two parameters nonlinear isotherm models were applied, with findings indicating that sorption data were like both linear and non-linear isotherms. Sorption data were found to be in excellent agreement with the Freundlich isotherm with R2 value (0.99). The sorption capacity of the sorbent was computed i.e. 312.2 mg/g. Thermodynamic characteristics were also computed. It was concluded that the sorption of methyl violet 10 B sorption on FB leaf powder is exothermic. Hence, it is a potentially cost-effective bio sorbent for exclusion of dye from wastewater.
Keywords: Exothermic process; Ficus benjamina; Freundlich isotherm; Methyl violet 10B; Nonlinear isotherms; Pseudo-second-order model; Sorption.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Achmad A., Kassim J., Kim Suan T., Che Amat R., Lean Seey T. Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from uncaria gambir extract. J. Phys. Sci. 2012;23(1):1–13.
-
- Enenebeaku C.E., Ukaga I.C., Okorocha J.N., Onyeachu B.I. Adsorption, Equilibrium and Kinetic Studies of the Removal of Methyl Violet from Aqueous Solution Using White Potato Peel Powder. 2018 doi: 10.18052/www.scipress.com/ILCPA.80.17. - DOI
-
- Ramakrishna K.R., Viraraghavan T. Dye removal using low cost adsorbents. Water Sci. Technol. Jan. 1997;36(2–3):189–196. doi: 10.1016/S0273-1223(97)00387-9. - DOI
-
- Lee J.W., Choi S.P., Thiruvenkatachari R., Shim W.G., Moon H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments. 2006;69(3):196–203. doi: 10.1016/J.DYEPIG.2005.03.008. - DOI
-
- Métivier-Pignon H., Faur-Brasquet C., Le Cloirec P. Adsorption of dyes onto activated carbon cloths: approach of adsorption mechanisms and coupling of ACC with ultrafiltration to treat coloured wastewaters. Sep. Purif. Technol. 2003;31(1):3–11. doi: 10.1016/S1383-5866(02)00147-8. Apr. - DOI
LinkOut - more resources
Full Text Sources

