Modulating Emission of Boric Acid into Highly Efficient and Color-Tunable Afterglow via Dehydration-Induced Through-Space Conjugation
- PMID: 36950728
- PMCID: PMC10214226
- DOI: 10.1002/advs.202300139
Modulating Emission of Boric Acid into Highly Efficient and Color-Tunable Afterglow via Dehydration-Induced Through-Space Conjugation
Abstract
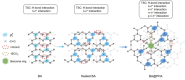
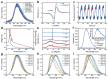
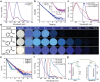
Inorganic boric acid (BA) is generally not considered an efficient afterglow material, and several groups have reported its extremely weak room-temperature phosphorescence (RTP) in the blue spectral region. It is discovered that heat treatment of BA results in increased afterglow intensity (27-fold increase) and prolonged emission lifetime (from 0.83 to 1.59 s), attributed to enhanced through-space conjugation (TSC) of BA. The afterglow intensity of BA can be increased further (≈415 folds) by introducing p-hydroxybenzoic acid (PHA), which contains a conjugated molecular motif, to further promote the TSC of the BA system. This combination results in the production of afterglow materials with a photoluminescence quantum yield of 83.8% and an emission lifetime of 2.01 s. In addition, a tunable multicolor afterglow in the 420-490 nm range is achieved owing to the enhancement of the RTP and thermally activated delayed fluorescence of PHA, where BA exerts a confinement effect on the guest molecules. Thus, this study demonstrates promising afterglow materials produced from extremely abundant and simple precursor materials for various applications.
Keywords: afterglow; boric acid; room-temperature phosphorescence; thermally activated delayed fluorescence; through-space conjugation.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhao W., He Z., Tang B. Z., Nat. Rev. Mater. 2020, 5, 869.
-
- Gao R., Kodaimati M. S., Yan D., Chem. Soc. Rev. 2021, 50, 5564. - PubMed
Grants and funding
- 22175052/National Natural Science Foundation of China
- 22171069/National Natural Science Foundation of China
- B2021201038/Science Fund for Creative Research Groups of Nature Science Foundation of Hebei Province
- B2020201060/Outstanding Youth Project of Natural Science Foundation of Hebei Province
- B2021202026/Natural Science Foundation of Hebei Province
LinkOut - more resources
Full Text Sources
