Patch repair protects cells from the small pore-forming toxin aerolysin
- PMID: 36951121
- PMCID: PMC10198622
- DOI: 10.1242/jcs.261018
Patch repair protects cells from the small pore-forming toxin aerolysin
Abstract
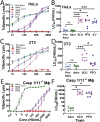
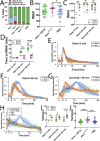
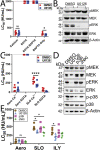
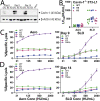
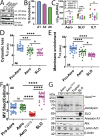
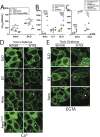
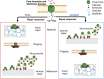
Aerolysin family pore-forming toxins damage the membrane, but membrane repair responses used to resist them, if any, remain controversial. Four proposed membrane repair mechanisms include toxin removal by caveolar endocytosis, clogging by annexins, microvesicle shedding catalyzed by MEK, and patch repair. Which repair mechanism aerolysin triggers is unknown. Membrane repair requires Ca2+, but it is controversial if Ca2+ flux is triggered by aerolysin. Here, we determined Ca2+ influx and repair mechanisms activated by aerolysin. In contrast to what is seen with cholesterol-dependent cytolysins (CDCs), removal of extracellular Ca2+ protected cells from aerolysin. Aerolysin triggered sustained Ca2+ influx. Intracellular Ca2+ chelation increased cell death, indicating that Ca2+-dependent repair pathways were triggered. Caveolar endocytosis failed to protect cells from aerolysin or CDCs. MEK-dependent repair did not protect against aerolysin. Aerolysin triggered slower annexin A6 membrane recruitment compared to CDCs. In contrast to what is seen with CDCs, expression of the patch repair protein dysferlin protected cells from aerolysin. We propose aerolysin triggers a Ca2+-dependent death mechanism that obscures repair, and the primary repair mechanism used to resist aerolysin is patch repair. We conclude that different classes of bacterial toxins trigger distinct repair mechanisms.
Keywords: Aeromonas; Annexin; Membrane repair; Patch repair; Pore-forming toxin; Streptolysin O.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
The fungal peptide toxin candidalysin induces distinct membrane repair mechanisms compared to bacterial pore-forming toxins.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653080. doi: 10.1101/2025.05.09.653080. bioRxiv. 2025. PMID: 40463135 Free PMC article. Preprint.
-
Aerolysin induces G-protein activation and Ca2+ release from intracellular stores in human granulocytes.J Biol Chem. 1998 Jul 17;273(29):18122-9. doi: 10.1074/jbc.273.29.18122. J Biol Chem. 1998. PMID: 9660770
-
Small Pore-Forming Toxins Different Membrane Area Binding and Ca2+ Permeability of Pores Determine Cellular Resistance of Monocytic Cells.Toxins (Basel). 2021 Feb 9;13(2):126. doi: 10.3390/toxins13020126. Toxins (Basel). 2021. PMID: 33572185 Free PMC article.
-
Cholesterol-dependent cytolysins.Adv Exp Med Biol. 2010;677:56-66. doi: 10.1007/978-1-4419-6327-7_5. Adv Exp Med Biol. 2010. PMID: 20687480 Review.
-
Branching out the aerolysin, ETX/MTX-2 and Toxin_10 family of pore forming proteins.J Invertebr Pathol. 2021 Nov;186:107570. doi: 10.1016/j.jip.2021.107570. Epub 2021 Mar 26. J Invertebr Pathol. 2021. PMID: 33775676 Review.
Cited by
-
The fungal peptide toxin candidalysin induces distinct membrane repair mechanisms compared to bacterial pore-forming toxins.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653080. doi: 10.1101/2025.05.09.653080. bioRxiv. 2025. PMID: 40463135 Free PMC article. Preprint.
-
The Multifaceted Role of Calcium Signaling in Regulated Necrosis.Biomolecules. 2025 Jun 11;15(6):854. doi: 10.3390/biom15060854. Biomolecules. 2025. PMID: 40563494 Free PMC article. Review.
-
Translational implications of targeting annexin A2: From membrane repair to muscular dystrophy, cardiovascular disease and cancer.Clin Transl Discov. 2023 Oct;3(5):e240. doi: 10.1002/ctd2.240. Epub 2023 Sep 5. Clin Transl Discov. 2023. PMID: 38465198 Free PMC article.
-
Vav2 is a master regulator of repair against bacterial pore-forming toxins.bioRxiv [Preprint]. 2025 Aug 1:2025.07.31.668026. doi: 10.1101/2025.07.31.668026. bioRxiv. 2025. PMID: 40766405 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

