Activating Transcription Factor 3 Stimulates Follicle-Stimulating Hormone-β Expression In Vitro But Is Dispensable for Follicle-Stimulating Hormone Production in Murine Gonadotropes In Vivo
- PMID: 36951304
- PMCID: PMC10282924
- DOI: 10.1210/endocr/bqad050
Activating Transcription Factor 3 Stimulates Follicle-Stimulating Hormone-β Expression In Vitro But Is Dispensable for Follicle-Stimulating Hormone Production in Murine Gonadotropes In Vivo
Abstract
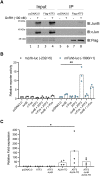
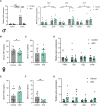
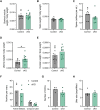
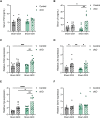
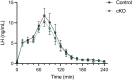
Follicle-stimulating hormone (FSH), a dimeric glycoprotein produced by pituitary gonadotrope cells, regulates spermatogenesis in males and ovarian follicle growth in females. Hypothalamic gonadotropin-releasing hormone (GnRH) stimulates FSHβ subunit gene (Fshb) transcription, though the underlying mechanisms are poorly understood. To address this gap in knowledge, we examined changes in pituitary gene expression in GnRH-deficient mice (hpg) treated with a regimen of exogenous GnRH that increases pituitary Fshb but not luteinizing hormone β (Lhb) messenger RNA levels. Activating transcription factor 3 (Atf3) was among the most upregulated genes. Activating transcription factor 3 (ATF3) can heterodimerize with members of the activator protein 1 family to regulate gene transcription. Co-expression of ATF3 with JunB stimulated murine Fshb, but not Lhb, promoter-reporter activity in homologous LβT2b cells. ATF3 also synergized with a constitutively active activin type I receptor to increase endogenous Fshb expression in these cells. Nevertheless, FSH production was intact in gonadotrope-specific Atf3 knockout [conditional knockout (cKO)] mice. Ovarian follicle development, ovulation, and litter sizes were equivalent between cKOs and controls. Testis weights and sperm counts did not differ between genotypes. Following gonadectomy, increases in LH secretion were enhanced in cKO animals. Though FSH levels did not differ between genotypes, post-gonadectomy increases in pituitary Fshb and gonadotropin α subunit expression were more pronounced in cKO than control mice. These data indicate that ATF3 can selectively stimulate Fshb expression in vitro but is not required for FSH production in vivo.
Keywords: GnRH; cell line; knockout mouse; pituitary; transcription.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
NR5A2 regulates Lhb and Fshb transcription in gonadotrope-like cells in vitro, but is dispensable for gonadotropin synthesis and fertility in vivo.PLoS One. 2013;8(3):e59058. doi: 10.1371/journal.pone.0059058. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23536856 Free PMC article.
-
Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity.Endocrinology. 2008 Nov;149(11):5577-91. doi: 10.1210/en.2008-0220. Epub 2008 Jul 24. Endocrinology. 2008. PMID: 18653705 Free PMC article.
-
Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model.Endocrinology. 2001 Jun;142(6):2284-95. doi: 10.1210/endo.142.6.8185. Endocrinology. 2001. PMID: 11356674
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
-
Rerouting of follicle-stimulating hormone secretion and gonadal function.Fertil Steril. 2023 Feb;119(2):180-183. doi: 10.1016/j.fertnstert.2022.12.005. Epub 2022 Dec 7. Fertil Steril. 2023. PMID: 36496082 Free PMC article. Review.
Cited by
-
Transcriptome analysis reveals pituitary lncRNA, circRNA and mRNA affecting fertility in high- and low-yielding goats.Front Genet. 2023 Dec 12;14:1303031. doi: 10.3389/fgene.2023.1303031. eCollection 2023. Front Genet. 2023. PMID: 38152654 Free PMC article.
References
-
- Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15(2):205‐206. - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201‐204. - PubMed
-
- Weiss J, Axelrod L, Whitcomb RW, Harris PE, Crowley WF, Jameson JL. Hypogonadism caused by a single amino acid substitution in the beta subunit of luteinizing hormone. N Engl J Med. 1992;326(3):179‐183. - PubMed
-
- Wildt L, Häusler A, Marshall G, et al. . Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376‐385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

