Identification of lthB, a Gene Encoding a Putative Glycosyltransferase Family 8 Protein Required for Leptothrix Sheath Formation
- PMID: 36951572
- PMCID: PMC10132092
- DOI: 10.1128/aem.01919-22
Identification of lthB, a Gene Encoding a Putative Glycosyltransferase Family 8 Protein Required for Leptothrix Sheath Formation
Abstract
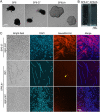
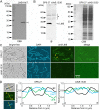
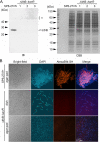
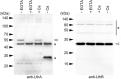
The bacterium Leptothrix cholodnii generates cell chains encased in sheaths that are composed of woven nanofibrils. The nanofibrils are mainly composed of glycoconjugate repeats, and several glycosyltransferases (GTs) are required for its biosynthesis. However, only one GT (LthA) has been identified to date. In this study, we screened spontaneous variants of L. cholodnii SP6 to find those that form smooth colonies, which is one of the characteristics of sheathless variants. Genomic DNA sequencing of an isolated variant revealed an insertion in the locus Lcho_0972, which encodes a putative GT family 8 protein. We thus designated this protein LthB and characterized it using deletion mutants and antibodies. LthB localized adjacent to the cell envelope. ΔlthB cell chains were nanofibril free and thus sheathless, indicating that LthB is involved in nanofibril biosynthesis. Unlike the ΔlthA mutant and the wild-type strain, which often generate planktonic cells, most ΔlthB organisms presented as long cell chains under static conditions, resulting in deficient pellicle formation, which requires motile planktonic cells. These results imply that sheaths are not required for elongation of cell chains. Finally, calcium depletion, which induces cell chain breakage due to sheath loss, abrogated the expression of LthA, but not LthB, suggesting that these GTs cooperatively participate in glycoconjugate biosynthesis under different signaling controls. IMPORTANCE In recent years, the regulation of cell chain elongation of filamentous bacteria via extracellular signals has attracted attention as a potential strategy to prevent clogging of water distribution systems and filamentous bulking of activated sludge in industrial settings. However, a fundamental understanding of the ecology of filamentous bacteria remains elusive. Since sheath formation is associated with cell chain elongation in most of these bacteria, the molecular mechanisms underlying nanofibril sheath formation, including the intracellular signaling cascade in response to extracellular stimuli, must be elucidated. Here, we isolated a sheathless variant of L. cholodnii SP6 and thus identified a novel glycosyltransferase, LthB. Although mutants with deletions of lthA, encoding another GT, and lthB were both defective for nanofibril formation, they exhibited different phenotypes of cell chain elongation and pellicle formation. Moreover, LthA expression, but not LthB expression, was influenced by extracellular calcium, which is known to affect nanofibril formation, indicating the functional diversities of LthA and LthB. Such molecular insights are critical for a better understanding of ecology of filamentous bacteria, which, in turn, can be used to improve strategies to control filamentous bacteria in industrial facilities.
Keywords: Leptothrix; filamentous bacterium; insertion sequence; next-generation sequencing; sheath formation; sheathless variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kvartenko O, Sabliy L, Kovalchuk N, Lysytsya A. 2018. The use of the biological method for treating iron containing underground waters. J Water Land Dev 39:77–82. 10.2478/jwld-2018-0061. - DOI
-
- Kunoh T, Kunoh H, Takada J. 2015. Perspectives on the biogenesis of iron oxide complexes produced by Leptothrix, an iron-oxidizing bacterium and promising industrial applications for their functions. J Microb Biochem Technol 7::419–426. 10.4172/1948-5948.1000249. - DOI
-
- Spring S. 2006. The genera Leptothrix and Sphaerotilus, p 758–777. In Prokaryotes: a handbook on the biology of bacteria, vol 5, 3rd ed. Springer, Berlin, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

