Activity of imipenem/relebactam against Enterobacterales and Pseudomonas aeruginosa in Spain. SMART 2016-2020
- PMID: 36951688
- PMCID: PMC10238800
- DOI: 10.37201/req/007.2023
Activity of imipenem/relebactam against Enterobacterales and Pseudomonas aeruginosa in Spain. SMART 2016-2020
Abstract
Objective: To determine susceptibility to the novel β-lactam/β-lactamase inhibitor combination imipenem/relebactam in clinical isolates recovered from intra-abdominal (IAI), urinary (UTI), respiratory (RTI) and bloodstream (BSI) infections in the SMART (Study for Monitoring Antimicrobial Resistance Trends) study in SPAIN during 2016 - 2020.
Methods: Broth microdilution MICs for imipenem/relebactam and comparators were determined by a central laboratory against isolates of Enterobacterales and Pseudomonas aeruginosa. MICs were interpreted using EUCAST-2021 breakpoints.
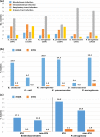
Results: In total, 5,210 Enterobacterales and 1,418 P. aeruginosa clinical isolates were analyzed. Imipenem/relebactam inhibited 98.8% of Enterobacterales. Distinguishing by source of infection susceptibility was 99.1% in BSI, 99.2% in IAI, 97.9% in RTI, and 99.2% in UTI. Of intensive care unit isolates (ICU) 97.4% were susceptible and of non-ICU isolates 99.2% were susceptible. In Enterobacterales, activity against Class A, Class B and Class D carbapenemases was 96.2%, 15.4% and 73.2%, respectively. In P. aeruginosa, imipenem/relebactam was active in 92.2% of isolates. By source of infection it was 94.8% in BSI, 92.9% in IAI, 91.7% in RTI, and 93.1% in UTI. An 88.7% of ICU isolates and 93.6% of non-ICU isolates were susceptible to imipenem/relebactam. Imipenem/relebactam remained active against P. aeruginosa ceftazidime-resistant (76.3%), cefepime-resistant (73.6%), imipenem-resistant (71.5%) and piperacillin-resistant (78.7%) isolates. Of all multidrug-resistant or difficult-to-treat resistance P. aeruginosa isolates, 75.1% and 46.2%, respectively, were susceptible to imipenem/relebactam.
Conclusions: Imipenem/relebactam showed high rates of susceptibility in Enterobacterales and P. aeruginosa isolates from different sources of infection as well as depending on patients' location (ICU or non-ICU scenarios).
Objetivos: Determinar la sensibilidad a la nueva combinación deβ-lactámico e inhibidor de β-lactamasas imipenem/ relebactam en aislados clínicos procedentes de infecciones intraabdominales (IIA), urinarias (ITU), respiratorias (ITR) y bacteriemias del estudio SMART (Study for Monitoring Antimicrobial Resistance Trends) en ESPAÑA durante 2016 - 2020.
Métodos: Se determinó la CMI mediante microdilución en caldo de imipenem/relebactam y antibióticos comparadores frente a aislados de Enterobacterales y Pseudomonas aeruginosa. Las CMI se analizaron empleando los puntos de corte EUCAST-2021.
Resultados: En total, se incluyeron 5.210 aislados de Enterobacterales y 1.418 aislados de P. aeruginosa. Imipenem/ relebactam fue activo frente al 98,8% de los Enterobacterales. Distinguiendo por foco de infección, la sensibilidad fue del 99,1% en bacteriemia, del 99,2% en IIA, del 97,9% en ITR y del 99,2% en ITU. El 97,4% de los aislados procedentes de unidades de cuidados intensivos (UCI) fueron sensibles, y el 99,2% de los aislados no procedentes de UCI. En Enterobacterales, la sensibilidad frente a carbapenemasas de clase A, clase B y clase D fue del 96,2%, 15,4% y 73,2%, respectivamente. En P. aeruginosa, imipenem/relebactam fue activo en el 92,2% de los aislados. Distinguiendo por foco de infección, la sensibilidad frente a P. aeruginosa fue del 94,8% en bacteriemia, 92,9% en IIA, 91,7% en ITR y 93,1% en ITU. El 88,7% de los aislados de la UCI y el 93,6% de los aislados no procedentes de UCI fueron sensibles a imipenem/relebactam. Imipenem/relebactam fue activo frente a aislados de P. aeruginosa resistentes a ceftazidima (76,3%), cefepima (73,6%), imipenem (71,5%) y piperacilina/tazobactam (78,7%). Frente a los aislados de P. aeruginosa clasificados como MDR o DTR, el 75,1% y el 46,2%, respectivamente, fueron sensibles a imipenem/relebactam.
Conclusiones: Imipenem/relebactam mostró elevada sensibilidad frente a los aislados de Enterobacterales y P. aeruginosa procedentes de diferentes focos de infección, así como en función de la localización de los pacientes (UCI o no UCI).
Keywords: Imipenem/relebactam; Intensive Care Unit; Multidrug-resistant; Spain; β-lactam/β-lactamase inhibitor combination.
©The Author 2023. Published by Sociedad Española de Quimioterapia. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)(https://creativecommons.org/licenses/by-nc/4.0/).
Conflict of interest statement
RC has participated in an educational program sponsored by GSK, Pfizer, MSD and Shionogi. RC has research grants funded by MSD, Shionogi and Venatorx. JDR and MG are employees of MSD Spain. All other authors declare no conflicts of interest.
Figures
Similar articles
-
Imipenem-Relebactam Susceptibility in Enterobacterales Isolates Recovered from ICU Patients from Spain and Portugal (SUPERIOR and STEP Studies).Microbiol Spectr. 2022 Oct 26;10(5):e0292722. doi: 10.1128/spectrum.02927-22. Epub 2022 Aug 31. Microbiol Spectr. 2022. PMID: 36043877 Free PMC article.
-
In vitro activity of imipenem/relebactam against piperacillin/tazobactam-resistant and meropenem-resistant non-Morganellaceae Enterobacterales and Pseudomonas aeruginosa collected from patients with bloodstream, intra-abdominal and urinary tract infections in Western Europe: SMART 2018-2020.J Med Microbiol. 2023 Feb;72(2). doi: 10.1099/jmm.0.001645. J Med Microbiol. 2023. PMID: 36763081
-
In vitro activity of imipenem-relebactam against resistant phenotypes of Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples - SMART Surveillance Europe 2015-2017.J Med Microbiol. 2020 Feb;69(2):207-217. doi: 10.1099/jmm.0.001142. Epub 2020 Jan 22. J Med Microbiol. 2020. PMID: 31976856
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
-
New Perspectives on Antimicrobial Agents: Imipenem-Relebactam.Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0025622. doi: 10.1128/aac.00256-22. Epub 2022 Jun 21. Antimicrob Agents Chemother. 2022. PMID: 35727059 Free PMC article. Review.
Cited by
-
Activity of cefepime, carbapenems and new β-lactam/β-lactamase inhibitor combinations on Enterobacter cloacae complex and Klebsiella aerogenes in Spain (SMART 2016-2022).JAC Antimicrob Resist. 2024 Jun 6;6(3):dlae087. doi: 10.1093/jacamr/dlae087. eCollection 2024 Jun. JAC Antimicrob Resist. 2024. PMID: 38847006 Free PMC article.
-
In-vitro activity of the novel β-lactam/β-lactamase inhibitor combinations and cefiderocol against carbapenem-resistant Pseudomonas spp. clinical isolates collected in Switzerland in 2022.Eur J Clin Microbiol Infect Dis. 2025 Mar;44(3):571-585. doi: 10.1007/s10096-024-04994-6. Epub 2024 Dec 20. Eur J Clin Microbiol Infect Dis. 2025. PMID: 39704920 Free PMC article.
References
-
- Tackling drug-resistant infections globally : final report and recommendations / the Review on Antimicrobial Resistance chaired by Jim O’Neill. doi: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c... Accessed 05 February 2023.
-
- Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al. . Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin Infect Dis. 2018;67(12):1803-1814. 10.1093/cid/ciy378. - DOI - PMC - PubMed
-
- Livermore DM, Nicolau DP, Hopkins KL, Meunier D. Carbapenem-Resistant Enterobacterales, Carbapenem Resistant Organisms, Carbapenemase-Producing Enterobacterales, and Carbapenemase-Producing Organisms: Terminology Past its “Sell-By Date” in an Era of New Antibiotics and Regional Carbapenemase Epidemiology. Clin Infect Dis. 2020;71:1776–82. 10.1093/cid/ciaa122 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

