Treatment With Liposomal Irinotecan Plus Fluorouracil and Leucovorin for Patients With Previously Treated Metastatic Biliary Tract Cancer: The Phase 2b NIFTY Randomized Clinical Trial
- PMID: 36951834
- PMCID: PMC10037199
- DOI: 10.1001/jamaoncol.2023.0016
Treatment With Liposomal Irinotecan Plus Fluorouracil and Leucovorin for Patients With Previously Treated Metastatic Biliary Tract Cancer: The Phase 2b NIFTY Randomized Clinical Trial
Abstract
Importance: The NIFTY trial demonstrated the benefit of treatment with second-line liposomal irinotecan (nal-IRI) plus fluorouracil (FU) and leucovorin (LV) for patients with advanced biliary tract cancer (BTC).
Objective: To report the updated efficacy outcomes from the NIFTY trial with extended follow-up of 1.3 years with reperformed masked independent central review (MICR) with 3 newly invited radiologists.
Design, setting, and participants: The NIFTY trial was a randomized, multicenter, open-label, phase 2b clinical trial conducted between September 5, 2018, and December 31, 2021, at 5 tertiary referral centers in South Korea. Patients with advanced BTC whose disease progressed while receiving first-line gemcitabine plus cisplatin with at least 1 measurable lesion per Response Evaluation Criteria in Solid Tumors, version 1.1, were eligible. Data analysis was completed on May 9, 2022.
Interventions: Patients were randomized 1:1 to receive LV, 400 mg/m2, bolus and FU, 2400 mg/m2, for a 46-hour infusion intravenously every 2 weeks with or without nal-IRI, 70 mg/m2, before LV intravenously. Patients were treated until disease progression or unacceptable toxic effects.
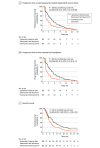
Main outcomes and measures: Primary end point was progression-free survival (PFS) as assessed by MICR. Secondary end points were PFS as assessed by the investigator, overall survival, and objective response rate.
Results: A total of 178 patients (75 women [42.1%]; median [IQR] age, 64 [38-84] years) were randomly assigned, and 174 patients were included in the full analysis set (88 patients [50.6%] in the nal-IRI plus FU/LV group vs 86 patients [49.4%] in the FU/LV alone group). In this updated analysis, the median MICR-assessed PFS was 4.2 months (95% CI, 2.8-5.3) for the nal-IRI plus FU/LV group and 1.7 months (95% CI, 1.4-2.6) for the FU/LV alone group (hazard ratio, 0.61; 95% CI, 0.44-0.86; P = .004), in contrast to the 7.1 and 1.4 months reported in the previous study, respectively. The discordance rate for tumor progression date between the MICR and investigators was 17.8% (vs 30% in the previous study).
Conclusions and relevance: The NIFTY randomized clinical trial demonstrated significant improvement in PFS with treatment with nal-IRI plus FU/LV compared with FU/LV alone for patients with advanced BTC after progression to gemcitabine plus cisplatin. The combination of nal-IRI plus FU/LV could be considered as a second-line treatment option for patients with previously treated advanced BTC.
Trial registration: clinicaltrials.gov Identifier: NCT03524508.
Conflict of interest statement
Figures