Preliminary Attainability Assessment of Real-World Data for Answering Major Clinical Research Questions in Breast Cancer Brain Metastasis: Framework Development and Validation Study
- PMID: 36951923
- PMCID: PMC10131620
- DOI: 10.2196/43359
Preliminary Attainability Assessment of Real-World Data for Answering Major Clinical Research Questions in Breast Cancer Brain Metastasis: Framework Development and Validation Study
Abstract
Background: In recent decades, real-world evidence (RWE) in oncology has rapidly gained traction for its potential to answer clinical questions that cannot be directly addressed by randomized clinical trials. Integrating real-world data (RWD) into clinical research promises to contribute to more sustainable research designs, including extension, augmentation, enrichment, and pragmatic designs. Nevertheless, clinical research using RWD is still limited because of concerns regarding the shortage of best practices for extracting, harmonizing, and analyzing RWD. In particular, pragmatic screening methods to determine whether the content of a data source is sufficient to answer the research questions before conducting the research with RWD have not yet been established.
Objective: We examined the PAR (Preliminary Attainability Assessment of Real-World Data) framework and assessed its utility in breast cancer brain metastasis (BCBM), which has an unmet medical need for data attainability screening at the preliminary step of observational studies that use RWD.
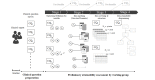
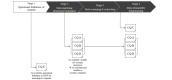
Methods: The PAR framework was proposed to assess data attainability from a particular data source during the early research process. The PAR framework has four sequential stages, starting with clinical question clarification: (1) operational definition of variables, (2) data matching (structural/semantic), (3) data screening and extraction, and (4) data attainability diagramming. We identified 5 clinical questions to be used for PAR framework evaluation through interviews and validated them with a survey of breast cancer experts. We used the Samsung Medical Center Breast Cancer Registry, a hospital-based real-time registry implemented in March 2021, leveraging the institution's anonymized and deidentified clinical data warehouse platform. The number of breast cancer patients in the registry was 45,129; it covered the period from June 1995 to December 2021. The registry consists of 24 base data marts that represent disease-specific breast cancer characteristics and care pathways. The outcomes included screening results of the clinical questions via the PAR framework and a procedural diagram of data attainability for each research question.
Results: Data attainability was tested for study feasibility according to the PAR framework with 5 clinical questions for BCBM. We obtained data sets that were sufficient to conduct studies with 4 of 5 clinical questions. The research questions stratified into 3 types when we developed data fields for clearly defined research variables. In the first, only 1 question could be answered using direct data variables. In the second, the other 3 questions required surrogate definitions that combined data variables. In the third, the question turned out to be not feasible for conducting further analysis.
Conclusions: The adoption of the PAR framework was associated with more efficient preliminary clinical research using RWD from BCBM. Furthermore, this framework helped accelerate RWE generation through clinical research by enhancing transparency and reproducibility and lowering the entry barrier for clinical researchers.
Keywords: PAR framework; brain metastasis; breast cancer; clinical data warehouse; observational study; preliminary attainability assessment; real-world data.
©Min Jeong Kim, Hyo Jung Kim, Danbee Kang, Hee Kyung Ahn, Soo-Yong Shin, Seri Park, Juhee Cho, Yeon Hee Park. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 23.03.2023.
Conflict of interest statement
Conflicts of Interest: YHP is a consultant or advisory board member for AstraZeneca, Pfizer, Lilly, MSD, Eisai, Roche, Daiichi-Sankyo, Menarini, Bixink, Everest, and Novartis Pharmaceuticals and received research funds from AstraZeneca, MSD, Pfizer, Novartis, and Roche. MJK is an employee of Roche Korea. HKA is a consultant for Daiichi Sankyo, Amgen, Yuhan, Novartis, Takeda, and Roche, and received payment for lectures from Bristol Myers Squibb, MSD, Eli Lilly, AstraZeneca, Boehringer Ingelheim, Menarini, Eisai, Pfizer, Boryung Pharmaceutical, Celltrion, Yuhan, and Pharmbio Korea. All other authors declare no conflicts of interest.
Figures


Similar articles
-
Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer.Eur J Cancer. 2023 Jun;186:52-61. doi: 10.1016/j.ejca.2023.03.013. Epub 2023 Mar 21. Eur J Cancer. 2023. PMID: 37030077
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Improving Transparency to Build Trust in Real-World Secondary Data Studies for Hypothesis Testing-Why, What, and How: Recommendations and a Road Map from the Real-World Evidence Transparency Initiative.Value Health. 2020 Sep;23(9):1128-1136. doi: 10.1016/j.jval.2020.04.002. Value Health. 2020. PMID: 32940229
-
Patient and public involvement and engagement in real world data and evidence research across the medicines development cycle: a rapid review of peer-reviewed literature and NICE technology appraisals.Curr Med Res Opin. 2025 Mar;41(3):521-533. doi: 10.1080/03007995.2025.2482668. Epub 2025 Mar 31. Curr Med Res Opin. 2025. PMID: 40126390 Review.
-
Trial designs using real-world data: The changing landscape of the regulatory approval process.Pharmacoepidemiol Drug Saf. 2020 Oct;29(10):1201-1212. doi: 10.1002/pds.4932. Epub 2019 Dec 10. Pharmacoepidemiol Drug Saf. 2020. PMID: 31823482 Free PMC article. Review.
Cited by
-
Transparency in the secondary use of health data: assessing the status quo of guidance and best practices.R Soc Open Sci. 2025 Mar 26;12(3):241364. doi: 10.1098/rsos.241364. eCollection 2025 Mar. R Soc Open Sci. 2025. PMID: 40144285 Free PMC article.
-
An Advanced Machine Learning Model for a Web-Based Artificial Intelligence-Based Clinical Decision Support System Application: Model Development and Validation Study.J Med Internet Res. 2024 Sep 4;26:e56022. doi: 10.2196/56022. J Med Internet Res. 2024. PMID: 39231422 Free PMC article.
-
Role of invasive mediastinal nodal staging in survival outcomes of patients with non-small cell lung cancer and without radiologic lymph node metastasis: a retrospective cohort study.EClinicalMedicine. 2024 Feb 10;69:102478. doi: 10.1016/j.eclinm.2024.102478. eCollection 2024 Mar. EClinicalMedicine. 2024. PMID: 38361994 Free PMC article.
References
-
- Pellerino A, Internò Valeria, Mo F, Franchino F, Soffietti R, Rudà Roberta. Management of brain and leptomeningeal metastases from breast cancer. Int J Mol Sci. 2020 Nov 12;21(22):8534. doi: 10.3390/ijms21228534. https://www.mdpi.com/resolver?pii=ijms21228534 ijms21228534 - DOI - PMC - PubMed
-
- Mills MN, Figura NB, Arrington JA, Yu HM, Etame AB, Vogelbaum MA, Soliman H, Czerniecki BJ, Forsyth PA, Han HS, Ahmed KA. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat. 2020 Apr;180(2):279–300. doi: 10.1007/s10549-020-05552-2.10.1007/s10549-020-05552-2 - DOI - PubMed
-
- Ahn HK, Park YH, Lee SJ, Park S, Maeng CH, Park W, Choi DH, Hur SJ, Ahn JS, Im Y. Clinical implication of Time To Brain Metastasis (TTBM) according to breast cancer subtypes. Springerplus. 2013 Dec;2(1):136. doi: 10.1186/2193-1801-2-136. https://europepmc.org/abstract/MED/23667803 225 - DOI - PMC - PubMed
-
- Joe NS, Hodgdon C, Kraemer L, Redmond KJ, Stearns V, Gilkes DM. A common goal to CARE: Cancer Advocates, Researchers, and Clinicians Explore current treatments and clinical trials for breast cancer brain metastases. NPJ Breast Cancer. 2021 Sep 14;7(1):121. doi: 10.1038/s41523-021-00326-5. doi: 10.1038/s41523-021-00326-5.10.1038/s41523-021-00326-5 - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

