IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models
- PMID: 36951942
- PMCID: PMC10145930
- DOI: 10.1172/JCI166028
IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models
Abstract
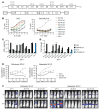
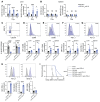
Patients with small cell lung cancer (SCLC) generally have a poor prognosis and a median overall survival of only about 13 months, indicating the urgent need for novel therapies. Delta-like protein 3 (DLL3) has been identified as a tumor-specific cell surface marker on neuroendocrine cancers, including SCLC. In this study, we developed a chimeric antigen receptor (CAR) against DLL3 that displays antitumor efficacy in xenograft and murine SCLC models. CAR T cell expression of the proinflammatory cytokine IL-18 greatly enhanced the potency of DLL3-targeting CAR T cell therapy. In a murine metastatic SCLC model, IL-18 production increased the activation of both CAR T cells and endogenous tumor-infiltrating lymphocytes. We also observed an increased infiltration, repolarization, and activation of antigen-presenting cells. Additionally, human IL-18-secreting anti-DLL3 CAR T cells showed an increased memory phenotype, less exhaustion, and induced durable responses in multiple SCLC models, an effect that could be further enhanced with anti-PD-1 blockade. All together, these results define DLL3-targeting CAR T cells that produce IL-18 as a potentially promising novel strategy against DLL3-expressing solid tumors.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Lung cancer; Oncology.
Conflict of interest statement
Figures



Comment in
-
Challenges and considerations in the immunotherapy of DLL3-positive small-cell lung cancer using IL-18 armoured chimeric antigen receptor T-cells.Transl Lung Cancer Res. 2024 Mar 29;13(3):678-683. doi: 10.21037/tlcr-23-793. Epub 2024 Mar 18. Transl Lung Cancer Res. 2024. PMID: 38601439 Free PMC article. No abstract available.
References
-
- Mahadevan NR, et al. Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. 2021;11(8):1952–1969. doi: 10.1158/2159-8290.CD-20-0913. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

