Durability of immune responses to mRNA booster vaccination against COVID-19
- PMID: 36951954
- PMCID: PMC10178835
- DOI: 10.1172/JCI167955
Durability of immune responses to mRNA booster vaccination against COVID-19
Abstract
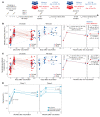
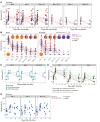
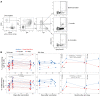
BackgroundMaintaining durable immunity following vaccination represents a major challenge, but whether mRNA booster vaccination improves durability is unknown.MethodsWe measured antibody responses in 55 healthy adults, who received a booster dose of the Pfizer-BioNTech or Moderna vaccine against SARS-CoV-2 and calculated the half-life of the antibody titers. We also measured memory B and T cell responses in a subset of 28 participants. In 13 volunteers who received a second booster vaccine, we measured serum antibody titers and memory B and T cell responses.ResultsThe booster (third immunization) dose at 6 to 10 months increased the half-life of the serum-neutralizing antibody (nAb) titers to 76 days from 56 to 66 days after the primary 2-dose vaccination. A second booster dose (fourth immunization) a year after the primary vaccination further increased the half-life to 88 days. However, despite this modestly improved durability in nAb responses against the ancestral (WA.1) strain, there was a loss of neutralization capacity against the Omicron subvariants BA.2.75.2, BQ.1.1, and XBB.1.5 (48-, 71-, and 66-fold drop in titers, respectively, relative to the WA.1 strain). Although only 45% to 65% of participants demonstrated a detectable nAb titer against the newer variants after the booster (third dose), the response declined to below the detection limit in almost all individuals by 6 months. In contrast, booster vaccination induced antigen-specific memory B and T cells that persisted for at least 6 months.ConclusionThe durability of serum antibody responses improves only marginally following booster immunizations with the Pfizer-BioNTech or Moderna mRNA vaccines.
Keywords: Adaptive immunity; Vaccines.
Figures

Update of
-
Durability of immune responses to the booster mRNA vaccination against COVID-19.medRxiv [Preprint]. 2022 Dec 4:2022.12.02.22282921. doi: 10.1101/2022.12.02.22282921. medRxiv. 2022. Update in: J Clin Invest. 2023 May 15;133(10):e167955. doi: 10.1172/JCI167955. PMID: 36482977 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

