Podocyte-specific deletion of ubiquitin carboxyl-terminal hydrolase L1 causes podocyte injury by inducing endoplasmic reticulum stress
- PMID: 36952018
- PMCID: PMC11073152
- DOI: 10.1007/s00018-023-04747-2
Podocyte-specific deletion of ubiquitin carboxyl-terminal hydrolase L1 causes podocyte injury by inducing endoplasmic reticulum stress
Abstract
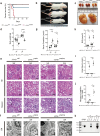
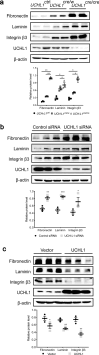
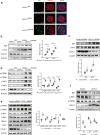
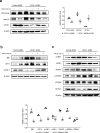
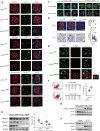
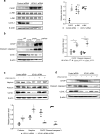
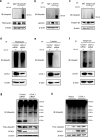
Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) is a unique component of the ubiquitin-proteasome system (UPS), which has multiple activities in maintaining intracellular ubiquitin levels. We previously reported the aberrant low expression of UCHL1 in podocytes of non-immune complex-mediated glomerulonephritis, and recent studies indicate that anti-UCHL1 antibody was responsible for the refractory minimal change disease (MCD), but the specific effect of UCHL1 to the podocytopathy has not been determined. Therefore, we generated podocyte-specific UCHL1 gene knockout (UCHL1cre/cre) rats model. Podocyte-specific UCHL1 knockout rats exhibited severe kidney damage, including segmental/global glomerulosclerosis, kidney function damage and severe proteinuria, compared with littermate control. Subsequently, by carrying out mass spectrometry analysis of isolated glomeruli of rats, abnormal protein accumulation of ECM-receptor Interaction was found in UCHL1cre/cre rats. Mechanistic studies in vivo and in vitro revealed that aberrant protein accumulation after UCHL1 deficiency induced endoplasmic reticulum (ER) stress, unfolded protein reaction (UPR) to reduce the protein level of podocyte skeleton proteins, and CHOP mediated apoptosis as well, which related to the dysfunction of the ubiquitin-proteasome system with decreased free monomeric ubiquitin level, thereby affecting protein ubiquitination and degradation. In addition, inhibition of ER stress by 4-PBA could attenuate the degree of ER stress and podocyte dysfunction. Our study indicates that UCHL1 is a potential target for preventing podocytes injury in some non-immune complex-mediated glomerulopathy.
Keywords: Endoplasmic reticulum stress; Podocyte apoptosis; Podocytopathy; Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1); Ubiquitin–proteasome system (UPS).
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Ubiquitin C-terminal hydrolase L1 deletion ameliorates glomerular injury in mice with ACTN4-associated focal segmental glomerulosclerosis.Biochim Biophys Acta. 2014 Jul;1842(7):1028-40. doi: 10.1016/j.bbadis.2014.03.009. Epub 2014 Mar 22. Biochim Biophys Acta. 2014. PMID: 24662305
-
Ubiquitin C-Terminal Hydrolase L1 is required for regulated protein degradation through the ubiquitin proteasome system in kidney.Kidney Int. 2018 Jan;93(1):110-127. doi: 10.1016/j.kint.2017.05.016. Epub 2017 Jul 26. Kidney Int. 2018. PMID: 28754552
-
UCH-L1 induces podocyte hypertrophy in membranous nephropathy by protein accumulation.Biochim Biophys Acta. 2014 Jul;1842(7):945-58. doi: 10.1016/j.bbadis.2014.02.011. Epub 2014 Feb 28. Biochim Biophys Acta. 2014. PMID: 24583340
-
Life and death in the trash heap: The ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia.Ageing Res Rev. 2017 Mar;34:30-38. doi: 10.1016/j.arr.2016.09.011. Epub 2016 Oct 1. Ageing Res Rev. 2017. PMID: 27702698 Free PMC article. Review.
-
The Intertwining of Autophagy and the Ubiquitin Proteasome System in Podocyte (Patho)Physiology.Cell Physiol Biochem. 2021 Sep 15;55(S4):68-95. doi: 10.33594/000000432. Cell Physiol Biochem. 2021. PMID: 34523304 Review.
Cited by
-
Oxysterol-binding protein-like 7 deficiency leads to ER stress-mediated apoptosis in podocytes and proteinuria.Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F340-F350. doi: 10.1152/ajprenal.00319.2023. Epub 2024 Jul 4. Am J Physiol Renal Physiol. 2024. PMID: 38961844
-
UCHL1 alleviates apoptosis in chondrocytes via upregulation of HIF‑1α‑mediated mitophagy.Int J Mol Med. 2023 Oct;52(4):99. doi: 10.3892/ijmm.2023.5302. Epub 2023 Sep 8. Int J Mol Med. 2023. PMID: 37681473 Free PMC article.
-
The Pathogenesis of Nephrotic Syndrome: A Perspective from B Cells.Kidney Dis (Basel). 2024 Aug 29;10(6):531-544. doi: 10.1159/000540511. eCollection 2024 Dec. Kidney Dis (Basel). 2024. PMID: 39664337 Free PMC article. Review.
-
The Life of a Kidney Podocyte.Acta Physiol (Oxf). 2025 Aug;241(8):e70081. doi: 10.1111/apha.70081. Acta Physiol (Oxf). 2025. PMID: 40698593 Free PMC article. Review.
-
High-mobility group box 1 in acute kidney injury.Front Pharmacol. 2025 Jul 14;16:1618971. doi: 10.3389/fphar.2025.1618971. eCollection 2025. Front Pharmacol. 2025. PMID: 40727103 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

