Vital Signs: Progress Toward Eliminating HIV as a Global Public Health Threat Through Scale-Up of Antiretroviral Therapy and Health System Strengthening Supported by the U.S. President's Emergency Plan for AIDS Relief - Worldwide, 2004-2022
- PMID: 36952290
- PMCID: PMC10042617
- DOI: 10.15585/mmwr.mm7212e1
Vital Signs: Progress Toward Eliminating HIV as a Global Public Health Threat Through Scale-Up of Antiretroviral Therapy and Health System Strengthening Supported by the U.S. President's Emergency Plan for AIDS Relief - Worldwide, 2004-2022
Abstract
Introduction: In 2004, the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), with CDC as a major U.S. government implementing agency, began providing HIV antiretroviral therapy (ART) worldwide. Through suppression of HIV viral load, effective ART reduces morbidity and mortality among persons with HIV infection and prevents vertical and sexual transmission.
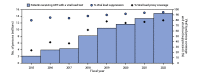
Methods: To describe program impact, data were analyzed from all PEPFAR programs and from six countries that have conducted nationally representative Population-based HIV Impact Assessment (PHIA) surveys, including PEPFAR programmatic data on the number of persons with HIV infection receiving PEPFAR-supported ART (2004-2022), rates of viral load coverage (the proportion of eligible persons with HIV infection who received a viral load test) and viral load suppression (proportion of persons who received a viral load test with <1,000 HIV copies per mL of blood) (2015-2022), and population viral load suppression rates in six countries that had two PHIA surveys conducted during 2015-2021. To assess health system strengthening, data on workforce and laboratory systems were analyzed.
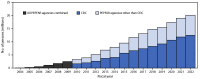
Results: By September 2022, approximately 20 million persons with HIV infection in 54 countries were receiving PEPFAR-supported ART (62% CDC-supported); this number increased 300-fold from the 66,550 reported in September 2004. During 2015-2022, viral load coverage more than tripled, from 24% to 80%, and viral load suppression increased from 80% to 95%. Despite increases in viral load suppression rates and health system strengthening investments, variability exists in viral load coverage among some subpopulations (children aged <10 years, males, pregnant women, men who have sex with men [MSM], persons in prisons and other closed settings [persons in prisons], and transgender persons) and in viral load suppression among other subpopulations (pregnant and breastfeeding women, persons in prisons, and persons aged <20 years).
Conclusions and implications for public health practice: Since 2004, PEPFAR has scaled up effective ART to approximately 20 million persons with HIV infection in 54 countries. To eliminate HIV as a global public health threat, achievements must be sustained and expanded to reach all subpopulations. CDC and PEPFAR remain committed to tackling HIV while strengthening public health systems and global health security.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Rodger AJ, Cambiano V, Bruun T, et al.; PARTNER Study Group. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–38. 10.1016/S0140-6736(19)30418-0 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

