Macrophage metabolic rewiring improves heme-suppressed efferocytosis and tissue damage in sickle cell disease
- PMID: 36952641
- PMCID: PMC10315632
- DOI: 10.1182/blood.2022018026
Macrophage metabolic rewiring improves heme-suppressed efferocytosis and tissue damage in sickle cell disease
Abstract
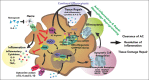
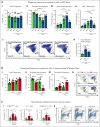
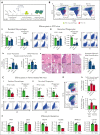
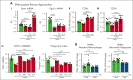
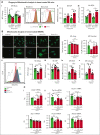
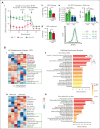
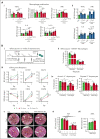
Sickle cell disease (SCD) is hallmarked by an underlying chronic inflammatory condition, which is contributed by heme-activated proinflammatory macrophages. Although previous studies addressed heme ability to stimulate macrophage inflammatory skewing through Toll-like receptor4 (TLR4)/reactive oxygen species signaling, how heme alters cell functional properties remains unexplored. Macrophage-mediated immune cell recruitment and apoptotic cell (AC) clearance are relevant in the context of SCD, in which tissue damage, cell apoptosis, and inflammation occur owing to vaso-occlusive episodes, hypoxia, and ischemic injury. Here we show that heme strongly alters macrophage functional response to AC damage by exacerbating immune cell recruitment and impairing cell efferocytic capacity. In SCD, heme-driven excessive leukocyte influx and defective efferocytosis contribute to exacerbated tissue damage and sustained inflammation. Mechanistically, these events depend on heme-mediated activation of TLR4 signaling and suppression of the transcription factor proliferator-activated receptor γ (PPARγ) and its coactivator peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α). These changes reduce efferocytic receptor expression and promote mitochondrial remodeling, resulting in a coordinated functional and metabolic reprogramming of macrophages. Overall, this results in limited AC engulfment, impaired metabolic shift to mitochondrial fatty acid β-oxidation, and, ultimately, reduced secretion of the antiinflammatory cytokines interleukin-4 (IL-4) and IL-10, with consequent inhibition of continual efferocytosis, resolution of inflammation, and tissue repair. We further demonstrate that impaired phagocytic capacity is recapitulated by macrophage exposure to plasma of patients with SCD and improved by hemopexin-mediated heme scavenging, PPARγ agonists, or IL-4 exposure through functional and metabolic macrophage rewiring. Our data indicate that therapeutic improvement of heme-altered macrophage functional properties via heme scavenging or PGC1α/PPARγ modulation significantly ameliorates tissue damage associated with SCD pathophysiology.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests. F.V. receives research funding from Silence Therapeutics, Vifor Pharma, and PharmaNutra (none of these are relevant to the current project).
Figures




Comment in
-
Heme and macrophages: a complicated liaison.Blood. 2023 Jun 22;141(25):3015-3017. doi: 10.1182/blood.2023020612. Blood. 2023. PMID: 37347498 No abstract available.
References
-
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572–2579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

