Cystic Fibrosis Reprograms Airway Epithelial IL-33 Release and Licenses IL-33-Dependent Inflammation
- PMID: 36952660
- PMCID: PMC10263140
- DOI: 10.1164/rccm.202211-2096OC
Cystic Fibrosis Reprograms Airway Epithelial IL-33 Release and Licenses IL-33-Dependent Inflammation
Abstract
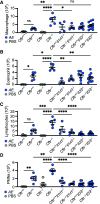
Rationale: Type 2 inflammation has been described in people with cystic fibrosis (CF). Whether loss of CFTR (cystic fibrosis transmembrane conductance regulator) function contributes directly to a type 2 inflammatory response has not been fully defined. Objectives: The potent alarmin IL-33 has emerged as a critical regulator of type 2 inflammation. We tested the hypothesis that CFTR deficiency increases IL-33 expression and/or release and deletion of IL-33 reduces allergen-induced inflammation in the CF lung. Methods: Human airway epithelial cells (AECs) grown from non-CF and CF cell lines and Cftr+/+ and Cftr-/- mice were used in this study. Pulmonary inflammation in Cftr+/+ and Cftr-/- mice with and without IL-33 or ST2 (IL-1 receptor-like 1) germline deletion was determined by histological analysis, BAL, and cytokine analysis. Measurements and Main Results: After allergen challenge, both CF human AECs and Cftr-/- mice had increased IL-33 expression compared with control AECs and Cftr+/+ mice, respectively. DUOX1 (dual oxidase 1) expression was increased in CF human AECs and Cftr-/- mouse lungs compared with control AECs and lungs from Cftr+/+ mice and was necessary for the increased IL-33 release in Cftr-/- mice compared with Cftr+/+ mice. IL-33 stimulation of Cftr-/- CD4+ T cells resulted in increased type 2 cytokine production compared with Cftr+/+ CD4+ T cells. Deletion of IL-33 or ST2 decreased both type 2 inflammation and neutrophil recruitment in Cftr-/- mice compared with Cftr+/+ mice. Conclusions: Absence of CFTR reprograms airway epithelial IL-33 release and licenses IL-33-dependent inflammation. Modulation of the IL-33/ST2 axis represents a novel therapeutic target in CF type 2-high and neutrophilic inflammation.
Keywords: adaptive immunity; cystic fibrosis transmembrane conductance regulator; immune system; innate immunity.
Figures





Comment in
-
An ALARMINg Type 2 Response in Cystic Fibrosis-The Key to Understanding ABPA?Am J Respir Crit Care Med. 2023 Jun 1;207(11):1418-1419. doi: 10.1164/rccm.202303-0580ED. Am J Respir Crit Care Med. 2023. PMID: 37023264 Free PMC article. No abstract available.
References
-
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med . 2011;184:75–81. - PubMed
-
- Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med . 1995;151:939–941. - PubMed
-
- Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros . 2015;14:419–430. - PubMed
-
- Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: an update. Pediatr Pulmonol . 2018;53:S30–S50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 HL159594/HL/NHLBI NIH HHS/United States
- R01 HL122554/HL/NHLBI NIH HHS/United States
- R38 HL143619/HL/NHLBI NIH HHS/United States
- R01 AI124456/AI/NIAID NIH HHS/United States
- R01 AI111820/AI/NIAID NIH HHS/United States
- I01 BX004299/BX/BLRD VA/United States
- R01 AI145265/AI/NIAID NIH HHS/United States
- R21 AI145397/AI/NIAID NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- K99 HL159594/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- T32 GM152284/GM/NIGMS NIH HHS/United States
- R01 HL136664/HL/NHLBI NIH HHS/United States
- U19 AI095227/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

