Hydrogen peroxide production by epidermal dual oxidase 1 regulates nociceptive sensory signals
- PMID: 36958249
- PMCID: PMC10038790
- DOI: 10.1016/j.redox.2023.102670
Hydrogen peroxide production by epidermal dual oxidase 1 regulates nociceptive sensory signals
Abstract
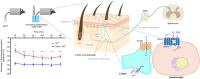
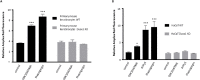
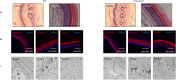
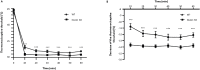
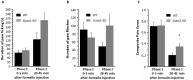
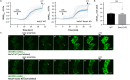
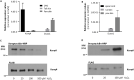
Keratinocytes of the mammalian skin provide not only mechanical protection for the tissues, but also transmit mechanical, chemical, and thermal stimuli from the external environment to the sensory nerve terminals. Sensory nerve fibers penetrate the epidermal basement membrane and function in the tight intercellular space among keratinocytes. Here we show that epidermal keratinocytes produce hydrogen peroxide upon the activation of the NADPH oxidase dual oxidase 1 (DUOX1). This enzyme can be activated by increasing cytosolic calcium levels. Using DUOX1 knockout animals as a model system we found an increased sensitivity towards certain noxious stimuli in DUOX1-deficient animals, which is not due to structural changes in the skin as evidenced by detailed immunohistochemical and electron-microscopic analysis of epidermal tissue. We show that DUOX1 is expressed in keratinocytes but not in the neural sensory pathway. The release of hydrogen peroxide by activated DUOX1 alters both the activity of neuronal TRPA1 and redox-sensitive potassium channels expressed in dorsal root ganglia primary sensory neurons. We describe hydrogen peroxide, produced by DUOX1 as a paracrine mediator of nociceptive signal transmission. Our results indicate that a novel, hitherto unknown redox mechanism modulates noxious sensory signals.
Keywords: DUOX1; Dual oxidase 1; Hydrogen peroxide; NADPH oxidase; Nociception; Skin.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflict of interest.
Figures

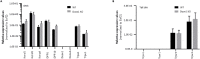

References
-
- Talagas M., Lebonvallet N., Leschiera R., Sinquin G., Elies P., Haftek M., Pennec J.P., Ressnikoff D., la Padula V., le Garrec R., L’herondelle K., Mignen O., le Pottier L., Kerfant N., Reux A., Marcorelles P., Misery L. Keratinocytes communicate with sensory neurons via synaptic-like contacts. Ann. Neurol. 2020;88 doi: 10.1002/ana.25912. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

