The intramembrane COOH-terminal domain of PRRT2 regulates voltage-dependent Na+ channels
- PMID: 36958475
- PMCID: PMC10164911
- DOI: 10.1016/j.jbc.2023.104632
The intramembrane COOH-terminal domain of PRRT2 regulates voltage-dependent Na+ channels
Abstract
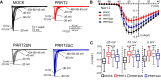
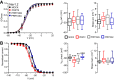
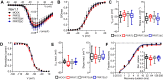
Proline-rich transmembrane protein 2 (PRRT2) is the single causative gene for pleiotropic paroxysmal syndromes, including epilepsy, kinesigenic dyskinesia, episodic ataxia, and migraine. PRRT2 is a neuron-specific type-2 membrane protein with a COOH-terminal intramembrane domain and a long proline-rich NH2-terminal cytoplasmic region. A large array of experimental data indicates that PRRT2 is a neuron stability gene that negatively controls intrinsic excitability by regulating surface membrane localization and biophysical properties of voltage-dependent Na+ channels Nav1.2 and Nav1.6, but not Nav1.1. To further investigate the regulatory role of PRRT2, we studied the structural features of this membrane protein with molecular dynamics simulations, and its structure-function relationships with Nav1.2 channels by biochemical and electrophysiological techniques. We found that the intramembrane COOH-terminal region maintains a stable conformation over time, with the first transmembrane domain forming a helix-loop-helix motif within the bilayer. The unstructured NH2-terminal cytoplasmic region bound to the Nav1.2 better than the isolated COOH-terminal intramembrane domain, mimicking full-length PRRT2, while the COOH-terminal intramembrane domain was able to modulate Na+ current and channel biophysical properties, still maintaining the striking specificity for Nav1.2 versus Nav1.1. channels. The results identify PRRT2 as a dual-domain protein in which the NH2-terminal cytoplasmic region acts as a binding antenna for Na+ channels, while the COOH-terminal membrane domain regulates channel exposure on the membrane and its biophysical properties.
Keywords: PRRT2; intrinsic excitability; molecular dynamics; structure-function relationships; voltage-dependent sodium channels.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Chen W.-J., Lin Y., Xiong Z.-Q., Wei W., Ni W., Tan G.-H., et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat. Genet. 2011;43:1252–1255. - PubMed
-
- Ebrahimi-Fakhari D., Saffari A., Westenberger A., Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain. 2015;138:3476–3495. - PubMed
-
- Heron S.E., Dibbens L.M. Role of PRRT2 in common paroxysmal neurological disorders: a gene with remarkable pleiotropy. J. Med. Genet. 2013;50:133–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

