Cardiopulmonary exercise testing during follow-up after acute pulmonary embolism
- PMID: 36958742
- PMCID: PMC10249018
- DOI: 10.1183/13993003.00059-2023
Cardiopulmonary exercise testing during follow-up after acute pulmonary embolism
Abstract
Background: Cardiopulmonary exercise testing (CPET) may provide prognostically valuable information during follow-up after pulmonary embolism (PE). Our objective was to investigate the association of patterns and degree of exercise limitation, as assessed by CPET, with clinical, echocardiographic and laboratory abnormalities and quality of life (QoL) after PE.
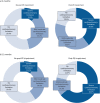
Methods: In a prospective cohort study of unselected consecutive all-comers with PE, survivors of the index acute event underwent 3- and 12-month follow-ups, including CPET. We defined cardiopulmonary limitation as ventilatory inefficiency or insufficient cardiocirculatory reserve. Deconditioning was defined as peak O2 uptake (V'O2 ) <80% with no other abnormality.
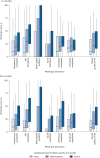
Results: Overall, 396 patients were included. At 3 months, prevalence of cardiopulmonary limitation and deconditioning was 50.1% (34.7% mild/moderate; 15.4% severe) and 12.1%, respectively; at 12 months, it was 44.8% (29.1% mild/moderate; 15.7% severe) and 14.9%, respectively. Cardiopulmonary limitation and its severity were associated with age (OR per decade 2.05, 95% CI 1.65-2.55), history of chronic lung disease (OR 2.72, 95% CI 1.06-6.97), smoking (OR 5.87, 95% CI 2.44-14.15) and intermediate- or high-risk acute PE (OR 4.36, 95% CI 1.92-9.94). Severe cardiopulmonary limitation at 3 months was associated with the prospectively defined, combined clinical-haemodynamic end-point of "post-PE impairment" (OR 6.40, 95% CI 2.35-18.45) and with poor disease-specific and generic health-related QoL.
Conclusions: Abnormal exercise capacity of cardiopulmonary origin is frequent after PE, being associated with clinical and haemodynamic impairment as well as long-term QoL reduction. CPET can be considered for selected patients with persisting symptoms after acute PE to identify candidates for closer follow-up and possible therapeutic interventions.
Copyright ©The authors 2023.
Conflict of interest statement
Conflicts of interest: S. Barco reports grants or contracts from Bayer, INARI, Boston Scientific, Medtronic, Bard, Sanofi and Concept Medical, consulting fees from INARI, payment or honoraria from INARI, Boston Scientific and Concept Medical, and support for attending meetings and/or travel from Bayer and Daiichi Sankyo. R. Ewert reports lecture fees from Boehringer Ingelheim, OMT, Novartis, Janssen-Cilag, United Therapeutics, AstraZeneca and Berlin-Chemie, research funding from Boehringer Ingelheim, OMT and Janssen-Cilag, and consulting fees from BetaPharm, OMT and Lungpacer Medical. G. Giannakoulas reports personal lecture/advisory fees from Bayer HealthCare, Pfizer and LeoPharma. L. Hobohm reports consulting fees and lecture honoraria from MSD and Janssen. S. Rosenkranz reports grants or contracts from Actelion, AstraZeneca, Bayer, Janssen and Novartis, consulting fees from Abbott, Acceleron, Actelion, Bayer, Janssen, MSD, Novartis, Pfizer, United Therapeutics and Vifor, and payment or honoraria from Actelion, Bayer, BMS, Ferrer, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, United Therapeutics and Vifor. T.A. Morris reports research funding from INARI. S.V. Konstantinides reports grants or contracts from Bayer AG, consulting fees from Bayer AG, Daiichi Sankyo and Boston Scientific, and payment or honoraria from Bayer AG, INARI Medical, MSD, Pfizer and BMS. M. Held reports honoraria for lectures and advisory board activities from AstraZeneca, Bayer HealthCare, Berlin-Chemie, Boehringer Ingelheim, BMS, Daichi Sankyo, Janssen, MSD, OMT, Pfizer and Santis. D. Dumitrescu reports honoraria for lectures or consulting fees from Actelion, Bayer, Boehringer Ingelheim Pharma, GlaxoSmithKline, Janssen, MSD, Novartis, OMT, Pfizer, Servier and Vifor. The remaining authors disclose no potential conflicts of interest.
Figures

References
-
- Barco S, Valerio L, Ageno W, et al. Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000–18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med 2021; 9: 33–42. doi: 10.1016/S2213-2600(20)30417-3 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
