Rituximab versus azathioprine for maintenance of remission for patients with ANCA-associated vasculitis and relapsing disease: an international randomised controlled trial
- PMID: 36958796
- PMCID: PMC10313987
- DOI: 10.1136/ard-2022-223559
Rituximab versus azathioprine for maintenance of remission for patients with ANCA-associated vasculitis and relapsing disease: an international randomised controlled trial
Abstract
Objective: Following induction of remission with rituximab in anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) relapse rates are high, especially in patients with history of relapse. Relapses are associated with increased exposure to immunosuppressive medications, the accrual of damage and increased morbidity and mortality. The RITAZAREM trial compared the efficacy of repeat-dose rituximab to daily oral azathioprine for prevention of relapse in patients with relapsing AAV in whom remission was reinduced with rituximab.
Methods: RITAZAREM was an international randomised controlled, open-label, superiority trial that recruited 188 patients at the time of an AAV relapse from 29 centres in seven countries between April 2013 and November 2016. All patients received rituximab and glucocorticoids to reinduce remission. Patients achieving remission by 4 months were randomised to receive rituximab intravenously (1000 mg every 4 months, through month 20) (85 patients) or azathioprine (2 mg/kg/day, tapered after month 24) (85 patients) and followed for a minimum of 36 months. The primary outcome was time to disease relapse (either major or minor relapse).
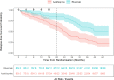
Results: Rituximab was superior to azathioprine in preventing relapse: HR 0.41; 95% CI 0.27 to 0.61, p<0.001. 19/85 (22%) patients in the rituximab group and 31/85 (36%) in the azathioprine group experienced at least one serious adverse event during the treatment period. There were no differences in rates of hypogammaglobulinaemia or infection between groups.
Conclusions: Following induction of remission with rituximab, fixed-interval, repeat-dose rituximab was superior to azathioprine for preventing disease relapse in patients with AAV with a prior history of relapse.
Trial registration number: NCT01697267; ClinicalTrials.gov identifier.
Keywords: B-lymphocytes; granulomatosis with polyangiitis; rituximab; systemic vasculitis; therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: RMS reports research grant for the trial from Roche during the conduct of the study. RBJ reports contract with Roche for provision of rituximab for another trial. US reports grants from Genentech, during the conduct of the study. AB has received consulting fees from AstraZeneca, Bayer, ChemoCentryx, Fresenius, and Vifor and honoraria for lectures from AstraZeneca, Bayer, ChemoCentryx, Fresenius, and Vifor. AB sits on the Advisory Board for AstraZeneca and Bayer and is Chair of the Immunonephrology working group of ERA. BC has received honoraria from Astra Zeneca and NAPP Pharmaceuticals. SC is on an advisory board for Sanofi. CKC reports participation in an advisory board for CSL Vifor. VD reports grants from NIDDK/NIH, NIAID/NIH and NHLBI/NIH; royalties from UptoDate; consulting fees from Novartis and Forma Therapeutics; participation on advisory boards for Merck, Forma Therapeutics and Bayer. LF reports grants from Bristol Meyer Squibb and Novartis and consulting fees from Chemocentryx. S Fujimoto reports grants and honoraria from Chugai Pharmaceutical Co., Ltd. S Furuta reports honoraria from Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo, Kissei Pharma, Asahi Kasei Pharma and Eisai. NK reports grants from BMS, Abbvie and Sanofi; consulting fees from Roche and honoraria from AstraZeneca, Kataka Medical, Otsuka, GlaxoSmithKline and Mallinckrodt. CK reports honoraria from Chemocentryx. CL reports grants from Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca and National Institutes of Health and honoraria from Ohio Association of Rheumatology, McGraw Hill, California Rheumatology Alliance and American Academy of Allergy, Asthma and Immunology. PL reports a research grant from Vifor pharma and consulting fees from Pfizer and is Co-chair, Rare Autoimmune Rheumatic Disease Alliance (RAIRDA), and Rare Diseases Clinical Lead, National Disease Registration Service, NHS Digital. PN has received consulting fees from Chemocentryx and Q32. C Pagnoux has received research grants from Teva, Amgen, Pfizer and Otsuka, consulting fees from Roche, Otsuka and GlaxoSmithKline and honoraria from Roche, GlaxoSmithKline, AstraZeneca and Otsuka and participated on an advisory board for AstraZeneca. C Pusey has received research grants from Kidney Research UK, Wellcome Trust, Medical Research Council, Vasculitis UK and Imperial Health Charity; consulting fees from Vifor and Alentis; honoraria from MedUpdate and Amgen; participation in advisory boards for COMBIVAS and OBIVAS trials. RS has received research grants from Roche-Genentech, AstraZeneca, GlaxoSmithKline, Kadmon, Boehringer Ingelheim, Chemocentryx, Corbus, Formation Biologics, Novartis, Inflarx and Principia; consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Regeneron, Abbvie, Sanofi, Chemocentryx, Novartis, Galderma, Vera, Chemomab and BMS; honoraria from GlaxoSmithKline, Abbvie, Sanofi, Chemocentryx, Novartis, Galderma and BMS. AS is an employee of Bristol-Myers Squibb. DJ has received grants from AstraZeneca, GlaxoSmithKline and Roche; consulting fees from Astra-Zeneca, Chemocentryx, GSK, Novartis, Otsuka, Takeda, Roche, Vifor; honoraria from GlaxoSmithKline and Vifor; participation on advisory boards for Chinook, GlaxoSmithKline and Takeda and stock options from Aurinia. PM reports research grants from AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, CSL Behring, Dynacure, EMDSerono, Forbius, GlaxoSmithKline, Immagene, InfalRx, Jannsen, Jubilant, Kyverna, Magenta, MicroBio, Neutrolis, Novartis, NS Pharma, Otsuka, Q32, Regeneron, Sparrow, Takeda; royalties from UpToDate; Consulting fees from AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, ChemoCentryx, CSL Behring, Dynacure, EMDSerono, Forbius, GlaxoSmithKline, immagene, InfalRx, Jannsen, Jubilant, Kyverna, Magenta, MicroBio, Neutrolis, Novartis, NS Pharma, Otsuka, Q32, Regeneron, Sparrow, Takeda; meeting fees from ChemoCentryx and stock options from Kyverna.
Figures

Comment in
-
In relapsing AAV with rituximab-induced remission, maintenance rituximab vs. azathioprine reduced relapse at ≥32 mo.Ann Intern Med. 2023 Aug;176(8):JC95. doi: 10.7326/J23-0057. Epub 2023 Aug 1. Ann Intern Med. 2023. PMID: 37523707
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

