Faecal metabolome and its determinants in inflammatory bowel disease
- PMID: 36958817
- PMCID: PMC10359577
- DOI: 10.1136/gutjnl-2022-328048
Faecal metabolome and its determinants in inflammatory bowel disease
Abstract
Objective: Inflammatory bowel disease (IBD) is a multifactorial immune-mediated inflammatory disease of the intestine, comprising Crohn's disease and ulcerative colitis. By characterising metabolites in faeces, combined with faecal metagenomics, host genetics and clinical characteristics, we aimed to unravel metabolic alterations in IBD.
Design: We measured 1684 different faecal metabolites and 8 short-chain and branched-chain fatty acids in stool samples of 424 patients with IBD and 255 non-IBD controls. Regression analyses were used to compare concentrations of metabolites between cases and controls and determine the relationship between metabolites and each participant's lifestyle, clinical characteristics and gut microbiota composition. Moreover, genome-wide association analysis was conducted on faecal metabolite levels.
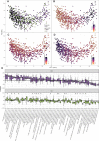
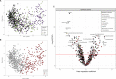
Results: We identified over 300 molecules that were differentially abundant in the faeces of patients with IBD. The ratio between a sphingolipid and L-urobilin could discriminate between IBD and non-IBD samples (AUC=0.85). We found changes in the bile acid pool in patients with dysbiotic microbial communities and a strong association between faecal metabolome and gut microbiota. For example, the abundance of Ruminococcus gnavus was positively associated with tryptamine levels. In addition, we found 158 associations between metabolites and dietary patterns, and polymorphisms near NAT2 strongly associated with coffee metabolism.
Conclusion: In this large-scale analysis, we identified alterations in the metabolome of patients with IBD that are independent of commonly overlooked confounders such as diet and surgical history. Considering the influence of the microbiome on faecal metabolites, our results pave the way for future interventions targeting intestinal inflammation.
Keywords: CROHN'S DISEASE; IBD; INTESTINAL MICROBIOLOGY; STOOL MARKERS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This study was funded by Takeda Development Center Americas. RKW acted as a consultant for Takeda and received unrestricted research grants from Takeda and Johnson and Johnson pharmaceuticals and speaker fees from AbbVie, MSD, Olympus and AstraZeneca. GA-A, CG, JS, JP and AAG are or were employees of Takeda Pharmaceuticals at the time this study was conducted.No disclosures: All other authors have nothing to disclose.
Figures





Comment in
- Gut. 2024 Jul 11;73(8):1392-1393. doi: 10.1136/gutjnl-2023-330196.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
