Sulfonium Salts as Acceptors in Electron Donor-Acceptor Complexes
- PMID: 36959098
- PMCID: PMC10952135
- DOI: 10.1002/anie.202303104
Sulfonium Salts as Acceptors in Electron Donor-Acceptor Complexes
Abstract
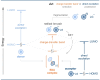
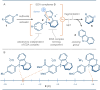
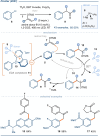
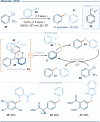
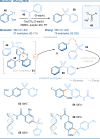
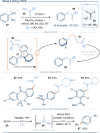
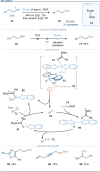
The photoactivation of electron donor-acceptor complexes has emerged as a sustainable, selective and versatile strategy for the generation of radical species. Electron donor-acceptor (EDA) complexation, however, imposes electronic constraints on the donor and acceptor components and this can limit the range of radicals that can be generated using the approach. New EDA complexation strategies exploiting sulfonium salts allow radicals to be generated from native functionality. For example, aryl sulfonium salts, formed by the activation of arenes, can serve as the acceptor components in EDA complexes due to their electron-deficient nature. This "sulfonium tag" approach relaxes the electronic constraints on the parent substrate and dramatically expands the range of radicals that can be generated using EDA complexation. In this review, these new applications of sulfonium salts will be introduced and the areas of chemical space rendered accessible through this innovation will be highlighted.
Keywords: Aryl Radical; Arylation; Charge Transfer; EDA Complex; Sulfonium Salt.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- DiRocco D. A., Dykstra K., Krska S., Vachal P., Conway D. V., Tudge M., Angew. Chem. Int. Ed. 2014, 53, 4802–4806; - PubMed
-
- None
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

