Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field
- PMID: 36959204
- PMCID: PMC10036336
- DOI: 10.1038/s41467-023-37225-1
Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field
Erratum in
-
Author Correction: Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field.Nat Commun. 2023 Jul 10;14(1):4067. doi: 10.1038/s41467-023-39906-3. Nat Commun. 2023. PMID: 37429881 Free PMC article. No abstract available.
Abstract
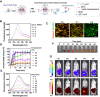
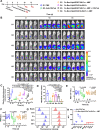
Micro-nano biorobots based on bacteria have demonstrated great potential for tumor diagnosis and treatment. The bacterial gene expression and drug release should be spatiotemporally controlled to avoid drug release in healthy tissues and undesired toxicity. Herein, we describe an alternating magnetic field-manipulated tumor-homing bacteria developed by genetically modifying engineered Escherichia coli with Fe3O4@lipid nanocomposites. After accumulating in orthotopic colon tumors in female mice, the paramagnetic Fe3O4 nanoparticles enable the engineered bacteria to receive and convert magnetic signals into heat, thereby initiating expression of lysis proteins under the control of a heat-sensitive promoter. The engineered bacteria then lyse, releasing its anti-CD47 nanobody cargo, that is pre-expressed and within the bacteria. The robust immunogenicity of bacterial lysate cooperates with anti-CD47 nanobody to activate both innate and adaptive immune responses, generating robust antitumor effects against not only orthotopic colon tumors but also distal tumors in female mice. The magnetically engineered bacteria also enable the constant magnetic field-controlled motion for enhanced tumor targeting and increased therapeutic efficacy. Thus, the gene expression and drug release behavior of tumor-homing bacteria can be spatiotemporally manipulated in vivo by a magnetic field, achieving tumor-specific CD47 blockage and precision tumor immunotherapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

