How autoreactive thymocytes differentiate into regulatory versus effector CD4+ T cells after avoiding clonal deletion
- PMID: 36959291
- PMCID: PMC10063450
- DOI: 10.1038/s41590-023-01469-2
How autoreactive thymocytes differentiate into regulatory versus effector CD4+ T cells after avoiding clonal deletion
Abstract
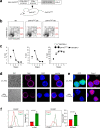
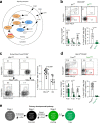
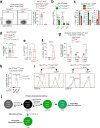
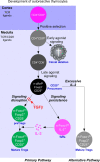
Thymocytes bearing autoreactive T cell receptors (TCRs) are agonist-signaled by TCR/co-stimulatory molecules to either undergo clonal deletion or to differentiate into specialized regulatory T (Treg) or effector T (Teff) CD4+ cells. How these different fates are achieved during development remains poorly understood. We now document that deletion and differentiation are agonist-signaled at different times during thymic selection and that Treg and Teff cells both arise after clonal deletion as alternative lineage fates of agonist-signaled CD4+CD25+ precursors. Disruption of agonist signaling induces CD4+CD25+ precursors to initiate Foxp3 expression and become Treg cells, whereas persistent agonist signaling induces CD4+CD25+ precursors to become IL-2+ Teff cells. Notably, we discovered that transforming growth factor-β induces Foxp3 expression and promotes Treg cell development by disrupting weaker agonist signals and that Foxp3 expression is not induced by IL-2 except under non-physiological in vivo conditions. Thus, TCR signaling disruption versus persistence is a general mechanism of lineage fate determination in the thymus that directs development of agonist-signaled autoreactive thymocytes.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

