Differentiation of patient-specific void urine-derived human induced pluripotent stem cells to fibroblasts and skeletal muscle myocytes
- PMID: 36959367
- PMCID: PMC10036466
- DOI: 10.1038/s41598-023-31780-9
Differentiation of patient-specific void urine-derived human induced pluripotent stem cells to fibroblasts and skeletal muscle myocytes
Abstract
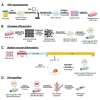
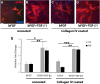
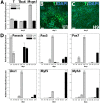
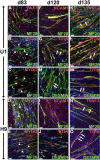
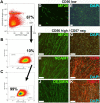
Cell-based therapy is a major focus for treatment of stress urinary incontinence (SUI). However, derivation of primary cells requires tissue biopsies, which often have adverse effects on patients. A recent study used human induced pluripotent stem cells (iPSC)-derived smooth muscle myocytes for urethral sphincter regeneration in rats. Here, we establish a workflow using iPSC-derived fibroblasts and skeletal myocytes for urethral tissue regeneration: (1) Cells from voided urine of women were reprogrammed into iPSC. (2) The iPSC line U1 and hESC line H9 (control) were differentiated into fibroblasts expressing FSP1, TE7, vinculin, vimentin, αSMA, fibronectin and paxillin. (3) Myogenic differentiation of U1 and H9 was induced by small molecule CHIR99021 and confirmed by protein expression of myogenic factors PAX7, MYOD, MYOG, and MF20. Striated muscle cells enriched by FACS expressed NCAM1, TITIN, DESMIN, TNNT3. (4) Human iPSC-derived fibroblasts and myocytes were engrafted into the periurethral region of RNU rats. Injected cells were labelled with ferric nanoparticles and traced by Prussian Blue stain, human-specific nuclear protein KU80, and human anti-mitochondria antibody. This workflow allows the scalable derivation, culture, and in vivo tracing of patient-specific fibroblasts and myocytes, which can be assessed in rat SUI models to regenerate urethral damages and restore continence.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

