A plant-produced SARS-CoV-2 spike protein elicits heterologous immunity in hamsters
- PMID: 36959936
- PMCID: PMC10028082
- DOI: 10.3389/fpls.2023.1146234
A plant-produced SARS-CoV-2 spike protein elicits heterologous immunity in hamsters
Abstract
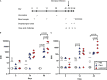
Molecular farming of vaccines has been heralded as a cheap, safe and scalable production platform. In reality, however, differences in the plant biosynthetic machinery, compared to mammalian cells, can complicate the production of viral glycoproteins. Remodelling the secretory pathway presents an opportunity to support key post-translational modifications, and to tailor aspects of glycosylation and glycosylation-directed folding. In this study, we applied an integrated host and glyco-engineering approach, NXS/T Generation™, to produce a SARS-CoV-2 prefusion spike trimer in Nicotiana benthamiana as a model antigen from an emerging virus. The size exclusion-purified protein exhibited a characteristic prefusion structure when viewed by transmission electron microscopy, and this was indistinguishable from the equivalent mammalian cell-produced antigen. The plant-produced protein was decorated with under-processed oligomannose N-glycans and exhibited a site occupancy that was comparable to the equivalent protein produced in mammalian cell culture. Complex-type glycans were almost entirely absent from the plant-derived material, which contrasted against the predominantly mature, complex glycans that were observed on the mammalian cell culture-derived protein. The plant-derived antigen elicited neutralizing antibodies against both the matched Wuhan and heterologous Delta SARS-CoV-2 variants in immunized hamsters, although titres were lower than those induced by the comparator mammalian antigen. Animals vaccinated with the plant-derived antigen exhibited reduced viral loads following challenge, as well as significant protection from SARS-CoV-2 disease as evidenced by reduced lung pathology, lower viral loads and protection from weight loss. Nonetheless, animals immunized with the mammalian cell-culture-derived protein were better protected in this challenge model suggesting that more faithfully reproducing the native glycoprotein structure and associated glycosylation of the antigen may be desirable.
Keywords: SARS-CoV-2; challenge; glycoprotein; glycosylation; immunogenicity; vaccine.
Copyright © 2023 Margolin, Schäfer, Allen, Gers, Woodward, Sutherland, Blumenthal, Meyers, Shaw, Preiser, Strasser, Crispin, Williamson, Rybicki and Chapman.
Conflict of interest statement
EM, AM, RC, A-LW, RS and ER have filed patent applications describing approaches to improve production of glycoproteins in plants. These include WO 2018/069878 A1, WO 2018/220595 A1, PA174002/PCT and PA176498_P. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

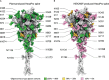

References
-
- Allen J. (2021). The glycan shields of HIV-1 and SARS-CoV-2 spike proteins and their differential importance in vaccine design. Doctor philosophy Univ. Southampton.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

