Degradation of collagen I by activated C1s in periodontal Ehlers-Danlos Syndrome
- PMID: 36960056
- PMCID: PMC10028100
- DOI: 10.3389/fimmu.2023.1157421
Degradation of collagen I by activated C1s in periodontal Ehlers-Danlos Syndrome
Abstract
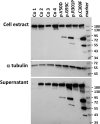
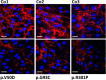
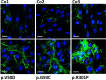
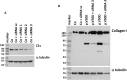
Periodontal Ehlers-Danlos syndrome (pEDS) is an autosomal dominant disorder characterized by early-onset periodontitis leading to premature loss of teeth, lack of attached gingiva and thin and fragile gums leading to gingival recession. Connective tissue abnormalities of pEDS typically include easy bruising, pretibial plaques, distal joint hypermobility, hoarse voice, and less commonly manifestations such as organ or vessel rupture. pEDS is caused by heterozygous missense mutations in C1R and C1S genes of the classical complement C1 complex. Previously we showed that pEDS pathogenic variants trigger intracellular activation of C1r and/or C1s, leading to extracellular presence of activated C1s. However, the molecular link relating activated C1r and C1s proteases to the dysregulated connective tissue homeostasis in pEDS is unknown. Using cell- and molecular-biological assays, we identified activated C1s (aC1s) as an enzyme which degrades collagen I in cell culture and in in vitro assays. Matrix collagen turnover in cell culture was assessed using labelled hybridizing peptides, which revealed fast and comprehensive collagen protein remodeling in patient fibroblasts. Furthermore, collagen I was completely degraded by aC1s when assays were performed at 40°C, indicating that even moderate elevated temperature has a tremendous impact on collagen I integrity. This high turnover is expected to interfere with the formation of a stable ECM and result in tissues with loose compaction a hallmark of the EDS phenotype. Our results indicate that pathogenesis in pEDS is not solely mediated by activation of the complement cascade but by inadequate C1s-mediated degradation of matrix proteins, confirming pEDS as a primary connective tissue disorder.
Keywords: C1r protease; Ehlers-Danlos Syndrome; collagen I; complement activation; periodontites.
Copyright © 2023 Amberger, Pertoll, Traunfellner, Kapferer-Seebacher, Stoiber, Klimaschewski, Thielens, Gaboriaud and Zschocke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
C1R Mutations Trigger Constitutive Complement 1 Activation in Periodontal Ehlers-Danlos Syndrome.Front Immunol. 2019 Nov 5;10:2537. doi: 10.3389/fimmu.2019.02537. eCollection 2019. Front Immunol. 2019. PMID: 31749804 Free PMC article.
-
Periodontal Ehlers-Danlos Syndrome Is Caused by Mutations in C1R and C1S, which Encode Subcomponents C1r and C1s of Complement.Am J Hum Genet. 2016 Nov 3;99(5):1005-1014. doi: 10.1016/j.ajhg.2016.08.019. Epub 2016 Oct 13. Am J Hum Genet. 2016. PMID: 27745832 Free PMC article.
-
Two Different Missense C1S Mutations, Associated to Periodontal Ehlers-Danlos Syndrome, Lead to Identical Molecular Outcomes.Front Immunol. 2019 Dec 18;10:2962. doi: 10.3389/fimmu.2019.02962. eCollection 2019. Front Immunol. 2019. PMID: 31921203 Free PMC article.
-
Periodontal manifestations of Ehlers-Danlos syndromes: A systematic review.J Clin Periodontol. 2017 Nov;44(11):1088-1100. doi: 10.1111/jcpe.12807. Epub 2017 Sep 25. J Clin Periodontol. 2017. PMID: 28836281
-
Vascular aspects of the Ehlers-Danlos Syndromes.Matrix Biol. 2018 Oct;71-72:380-395. doi: 10.1016/j.matbio.2018.04.013. Epub 2018 Apr 27. Matrix Biol. 2018. PMID: 29709596 Review.
Cited by
-
Impact of a Heterozygous C1RR301P/WT Mutation on Collagen Metabolism and Inflammatory Response in Human Gingival Fibroblasts.Cells. 2025 Mar 22;14(7):479. doi: 10.3390/cells14070479. Cells. 2025. PMID: 40214433 Free PMC article.
-
Characteristics of brittle cornea syndrome by multimodal imaging modalities: a case report.BMC Ophthalmol. 2023 Sep 15;23(1):378. doi: 10.1186/s12886-023-03123-9. BMC Ophthalmol. 2023. PMID: 37710225 Free PMC article.
-
Genetic diagnosis of the Ehlers-Danlos syndromes.Med Genet. 2024 Dec 3;36(4):235-245. doi: 10.1515/medgen-2024-2061. eCollection 2024 Dec. Med Genet. 2024. PMID: 39629471 Free PMC article.
-
The Successful Treatment of a Patient with Ehlers-Danlos Syndrome (EDS) After an Extensive Burn Injury: A Case Report.Medicina (Kaunas). 2025 Mar 21;61(4):554. doi: 10.3390/medicina61040554. Medicina (Kaunas). 2025. PMID: 40282845 Free PMC article.
References
-
- Kapferer-Seebacher I, Pepin M, Werner R, Aitman TJ, Nordgren A, Stoiber H, et al. . Periodontal ehlers-danlos syndrome is caused by mutations in C1R and C1S, which encode subcomponents C1r and C1s of complement. Am J Hum Genet (2016) 99(5):1005–14. doi: 10.1016/j.ajhg.2016.08.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

