Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions
- PMID: 36960064
- PMCID: PMC10028191
- DOI: 10.3389/fimmu.2023.1138215
Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions
Erratum in
-
Corrigendum: Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions.Front Immunol. 2025 May 5;16:1594972. doi: 10.3389/fimmu.2025.1594972. eCollection 2025. Front Immunol. 2025. PMID: 40391227 Free PMC article.
Abstract
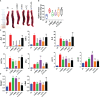
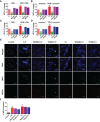
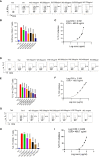
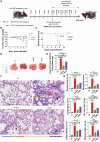
Cytokine release syndrome (CRS) due to severe acute respiratory coronavirus-2 (SARS-CoV-2) infection leads to life-threatening pneumonia which has been associated with coronavirus disease (COVID-19) pathologies. Centuries-old Asian traditional medicines such as Withania somnifera (L.) Dunal (WS) and Tinospora cordifolia (Willd.) Miers (TC) possess potent immunomodulatory effects and were used by the AYUSH ministry, in India during the COVID-19 pandemic. In the present study, we investigated WS and TC's anti-viral and immunomodulatory efficacy at the human equivalent doses using suitable in vitro and in vivo models. While both WS and TC showed immuno-modulatory potential, WS showed robust protection against loss in body weight, viral load, and pulmonary pathology in the hamster model of SARS-CoV2. In vitro pretreatment of mice and human neutrophils with WS and TC had no adverse effect on PMA, calcium ionophore, and TRLM-induced ROS generation, phagocytosis, bactericidal activity, and NETs formation. Interestingly, WS significantly suppressed the pro-inflammatory cytokines-induced Th1, Th2, and Th17 differentiation. We also used hACE2 transgenic mice to further investigate the efficacy of WS against acute SARS-CoV2 infection. Prophylactic treatment of WS in the hACE2 mice model showed significant protection against body weight loss, inflammation, and the lung viral load. The results obtained indicate that WS promoted the immunosuppressive environment in the hamster and hACE2 transgenic mice models and limited the worsening of the disease by reducing inflammation, suggesting that WS might be useful against other acute viral infections. The present study thus provides pre-clinical efficacy data to demonstrate a robust protective effect of WS against COVID-19 through its broader immunomodulatory activity.
Keywords: COVID-19; SARS-CoV-2; T cells; Tinospora cordifolia; Withania somnifera (Ashwagandha); and hACE2 transgenic mice; hamster model; neutrophils.
Copyright © 2023 Rizvi, Babele, Madan, Sadhu, Tripathy, Goswami, Mani, Dikshit and Awasthi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Corrigendum: Pharmacological potential of Withania somnifera (L.) Dunal and Tinospora cordifolia (Willd.) Miers on the experimental models of COVID-19, T cell differentiation, and neutrophil functions.Front Immunol. 2025 May 5;16:1594972. doi: 10.3389/fimmu.2025.1594972. eCollection 2025. Front Immunol. 2025. PMID: 40391227 Free PMC article.
-
Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study.J Biomol Struct Dyn. 2022 Jan;40(1):190-203. doi: 10.1080/07391102.2020.1810778. Epub 2020 Aug 27. J Biomol Struct Dyn. 2022. PMID: 32851919 Free PMC article.
-
Withania somnifera (L.) Dunal (Ashwagandha) for the possible therapeutics and clinical management of SARS-CoV-2 infection: Plant-based drug discovery and targeted therapy.Front Cell Infect Microbiol. 2022 Aug 15;12:933824. doi: 10.3389/fcimb.2022.933824. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36046742 Free PMC article. Review.
-
The Plausible Role of Indian Traditional Medicine in Combating Corona Virus (SARS-CoV 2): A Mini-Review.Curr Pharm Biotechnol. 2021;22(7):906-919. doi: 10.2174/1389201021666200807111359. Curr Pharm Biotechnol. 2021. PMID: 32767920 Review.
-
Dry leaf extracts of Tinospora cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells.Phytomedicine. 2019 Aug;61:152831. doi: 10.1016/j.phymed.2019.152831. Epub 2019 Jan 10. Phytomedicine. 2019. PMID: 31035042
Cited by
-
Evaluation of Ayush-64 (a Polyherbal Formulation) and Its Ingredients in the Syrian Hamster Model for SARS-CoV-2 Infection Reveals the Preventative Potential of Alstonia scholaris.Pharmaceuticals (Basel). 2023 Sep 21;16(9):1333. doi: 10.3390/ph16091333. Pharmaceuticals (Basel). 2023. PMID: 37765142 Free PMC article.
-
The aqueous root extract of Withania somnifera ameliorates LPS-induced inflammatory changes in the in vitro cell-based and mice models of inflammation.Front Pharmacol. 2023 Jun 12;14:1139654. doi: 10.3389/fphar.2023.1139654. eCollection 2023. Front Pharmacol. 2023. PMID: 37377934 Free PMC article.
-
Tinospora cordifolia (Giloy): An insight on the multifarious pharmacological paradigms of a most promising medicinal ayurvedic herb.Heliyon. 2024 Feb 15;10(4):e26125. doi: 10.1016/j.heliyon.2024.e26125. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38390130 Free PMC article. Review.
-
Berbamine prevents SARS-CoV-2 entry and transmission.iScience. 2024 Nov 8;27(12):111347. doi: 10.1016/j.isci.2024.111347. eCollection 2024 Dec 20. iScience. 2024. PMID: 39640591 Free PMC article.
-
Mycobacterium tuberculosis strain with deletions in menT3 and menT4 is attenuated and confers protection in mice and guinea pigs.Nat Commun. 2024 Jun 27;15(1):5467. doi: 10.1038/s41467-024-49246-5. Nat Commun. 2024. PMID: 38937463 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

