Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection
- PMID: 36960070
- PMCID: PMC10027912
- DOI: 10.3389/fimmu.2023.1131229
Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection
Abstract
Background: Whether or not a single-dose Ad26.COV2.S prime and boost vaccination induces sufficient immunity is unclear. Concerns about the increased risk of breakthrough infections in the Ad26.COV2.S-primed population have also been raised.
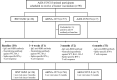
Methods: A prospective cohort study was conducted. Participants included healthy adults who were Ad26.COV2.S primed and scheduled to receive a booster vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S. The IgG anti-receptor binding domain (RBD) antibody titers, neutralizing antibody (NAb) titers (against wild type [WT] and Omicron [BA.1 and BA.5]), and Spike-specific interferon-γ responses of the participants were estimated at baseline, 3-4 weeks, 3 months, and 6 months after booster vaccination.
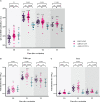
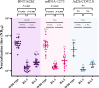
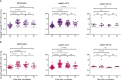
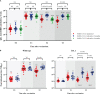
Results: A total of 89 participants were recruited (26 boosted with BNT162b2, 57 with mRNA-1273, and 7 with Ad26.COV2.S). The IgG anti-RBD antibody titers of all participants were significantly higher at 6 months post-vaccination than at baseline. The NAb titers against WT at 3 months post-vaccination were 359, 258, and 166 in the participants from the BNT162b2-, mRNA-1273-, and Ad26.COV2.S-boosted groups, respectively. Compared with those against WT, the NAb titers against BA.1/BA.5 were lower by 23.9/10.9-, 16.6/7.4-, and 13.8/7.2-fold in the participants from the BNT162b2-, mRNA-1273-, and Ad26.COV2.S-boosted groups, respectively, at 3 months post-vaccination. Notably, the NAb titers against BA.1 were not boosted after Ad26.COV2.S vaccination. Breakthrough infections occurred in 53.8%, 62.5%, and 42.9% of the participants from the BNT162b2-, mRNA-1273-, and Ad26.COV2.S-boosted groups, respectively. No significant difference in humoral and cellular immunity was found between individuals with and without SARS-CoV-2 breakthrough infections.
Conclusion: Booster vaccination elicited acceptable humoral and cellular immune responses in Ad26.COV2.S-primed individuals. However, the neutralizing activities against Omicron subvariants were negligible, and breakthrough infection rates were remarkably high at 3 months post-booster vaccination, irrespective of the vaccine type. A booster dose of a vaccine containing the Omicron variant antigen would be required.
Keywords: COVID-19; SARS-CoV-2; booster; breakthrough infection; cellular immunity; humoral immunity; vaccines.
Copyright © 2023 Hyun, Jang, Park, Heo, Seo, Nham, Yoon, Seong, Noh, Cheong, Kim, Yoon, Seok, Kim, Park and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Immunogenicity of bivalent omicron (BA.1) booster vaccination after different priming regimens in health-care workers in the Netherlands (SWITCH ON): results from the direct boost group of an open-label, multicentre, randomised controlled trial.Lancet Infect Dis. 2023 Aug;23(8):901-913. doi: 10.1016/S1473-3099(23)00140-8. Epub 2023 Apr 21. Lancet Infect Dis. 2023. PMID: 37088096 Free PMC article. Clinical Trial.
-
Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers.Int J Infect Dis. 2022 Oct;123:183-191. doi: 10.1016/j.ijid.2022.08.022. Epub 2022 Aug 28. Int J Infect Dis. 2022. PMID: 36044963 Free PMC article.
-
Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming.N Engl J Med. 2022 Mar 10;386(10):951-963. doi: 10.1056/NEJMoa2116747. Epub 2022 Jan 19. N Engl J Med. 2022. PMID: 35045226 Free PMC article. Clinical Trial.
-
Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
-
Longevity of immunity following COVID-19 vaccination: a comprehensive review of the currently approved vaccines.Hum Vaccin Immunother. 2022 Nov 30;18(5):2037384. doi: 10.1080/21645515.2022.2037384. Epub 2022 Apr 13. Hum Vaccin Immunother. 2022. PMID: 35417285 Free PMC article. Review.
Cited by
-
Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations.Vaccines (Basel). 2023 Aug 4;11(8):1320. doi: 10.3390/vaccines11081320. Vaccines (Basel). 2023. PMID: 37631890 Free PMC article.
-
Comparative duration of neutralizing responses and protections of COVID-19 vaccination and correlates of protection.Nat Commun. 2025 May 22;16(1):4748. doi: 10.1038/s41467-025-60024-9. Nat Commun. 2025. PMID: 40404724 Free PMC article.
-
Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: a cohort study from the Fukushima vaccination community survey, Japan.Front Immunol. 2023 Aug 16;14:1240425. doi: 10.3389/fimmu.2023.1240425. eCollection 2023. Front Immunol. 2023. PMID: 37662950 Free PMC article.
-
Humoral and cellular immune durability of different COVID-19 vaccine platforms following homologous/heterologous boosters: one-year post vaccination.Front Immunol. 2025 Jan 22;16:1526444. doi: 10.3389/fimmu.2025.1526444. eCollection 2025. Front Immunol. 2025. PMID: 39911379 Free PMC article.
-
COVID-19 Vaccine Booster Dose Fails to Enhance Antibody Response to Omicron Variant in Reinfected Healthcare Workers.Viruses. 2025 Jan 9;17(1):78. doi: 10.3390/v17010078. Viruses. 2025. PMID: 39861867 Free PMC article.
References
-
- Bartsch YC, Tong X, Kang J, Avendaño MJ, Serrano EF, García-Salum T, et al. . Omicron variant spike-specific antibody binding and fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines. Sci Transl Med (2022) 14:eabn9243. doi: 10.1126/scitranslmed.abn9243 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

