The prognostic significance of human ovarian aging-related signature in breast cancer after surgery: A multicohort study
- PMID: 36960071
- PMCID: PMC10027938
- DOI: 10.3389/fimmu.2023.1139797
The prognostic significance of human ovarian aging-related signature in breast cancer after surgery: A multicohort study
Abstract
Background: Recent studies have shown that ovarian aging is strongly associated with the risk of breast cancer, however, its prognostic impact on breast cancer is not yet fully understood. In this study, we performed a multicohort genetic analysis to explore its prognostic value and biological features in breast cancer.
Methods: The gene expression and clinicopathological data of 3366 patients from the The Cancer Genome Atlas (TCGA) cohort, the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort and the GSE86166 cohort were analyzed. A total of 290 ovarian aging-related genes (OARGs) were included in the establishment of the prognostic model. Furthermore, functional mechanisms analysis, drug sensitivity, and immune cell infiltration were investigated using bioinformatic methods.
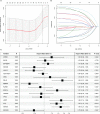
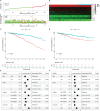
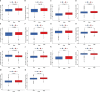
Results: An eight OARG-based signature was established and validated using independent cohorts. Two risk subgroups of patients with distinct survival outcomes were identified by the OARG-based signature. A nomogram with good predictive performance was developed by integrating the OARG risk score with clinicopathological factors. Moreover, the OARG-based signature was correlated with DNA damage repair, immune cell signaling pathways, and immunomodulatory functions. The patients in the low-risk subgroup were found to be sensitive to traditional chemotherapeutic, endocrine, and targeted agents (doxorubicin, tamoxifen, lapatinib, etc.) and some novel targeted drugs (sunitinib, pazopanib, etc.). Moreover, patients in the low-risk subgroup may be more susceptible to immune escape and therefore respond less effectively to immunotherapy.
Conclusions: In this study, we proposed a comprehensive analytical method for breast cancer assessment based on OARG expression patterns, which could precisely predict clinical outcomes and drug sensitivity of breast cancer patients.
Keywords: breast cancer; drug sensitivity; immune infiltration; ovarian ageing; prognosis.
Copyright © 2023 Hua, Zhu, Zhang, Cao, Wang, Gao and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Collaborative Group on Hormonal Factors in Breast C . Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol (2012) 13(11):1141–51. doi: 10.1016/S1470-2045(12)70425-4 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

