Structural and ligand binding analysis of the pet allergens Can f 1 and Fel d 7
- PMID: 36960093
- PMCID: PMC10028261
- DOI: 10.3389/falgy.2023.1133412
Structural and ligand binding analysis of the pet allergens Can f 1 and Fel d 7
Abstract
Introduction: Pet lipocalins are respiratory allergens with a central hydrophobic ligand-binding cavity called a calyx. Molecules carried in the calyx by allergens are suggested to influence allergenicity, but little is known about the native ligands.
Methods: To provide more information on prospective ligands, we report crystal structures, NMR, molecular dynamics, and florescence studies of a dog lipocalin allergen Can f 1 and its closely related (and cross-reactive) cat allergen Fel d 7.
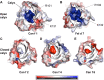
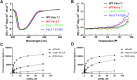
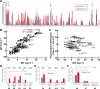
Results: Structural comparisons with reported lipocalins revealed that Can f 1 and Fel d 7 calyxes are open and positively charged while other dog lipocalin allergens are closed and negatively charged. We screened fatty acids as surrogate ligands, and found that Can f 1 and Fel d 7 bind multiple ligands with preferences for palmitic acid (16:0) among saturated fatty acids and oleic acid (18:1 cis-9) among unsaturated ones. NMR analysis of methyl probes reveals that conformational changes occur upon binding of pinolenic acid inside the calyx. Molecular dynamics simulation shows that the carboxylic group of fatty acids shuttles between two positively charged amino acids inside the Can f 1 and Fel d 7 calyx. Consistent with simulations, the stoichiometry of oleic acid-binding is 2:1 (fatty acid: protein) for Can f 1 and Fel d 7.
Discussion: The results provide valuable insights into the determinants of selectivity and candidate ligands for pet lipocalin allergens Can f 1 and Fel d 7.
Keywords: allergen; cat; dog; lipocalin; structure.
© 2023 Min, Foo, Gabel, Perera, DeRose, Pomés, Pedersen and Mueller.
Conflict of interest statement
AP is employed by InBio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor CH declared a past co-authorship with the author AP.
Figures



Similar articles
-
The cat lipocalin Fel d 7 and its cross-reactivity with the dog lipocalin Can f 1.Allergy. 2016 Oct;71(10):1490-5. doi: 10.1111/all.12955. Epub 2016 Jul 11. Allergy. 2016. PMID: 27289080
-
Crystal structure of the dog allergen Can f 6 and structure-based implications of its cross-reactivity with the cat allergen Fel d 4.Sci Rep. 2019 Feb 6;9(1):1503. doi: 10.1038/s41598-018-38134-w. Sci Rep. 2019. PMID: 30728436 Free PMC article.
-
Crystal structure of the dog lipocalin allergen Can f 2: implications for cross-reactivity to the cat allergen Fel d 4.J Mol Biol. 2010 Aug 6;401(1):68-83. doi: 10.1016/j.jmb.2010.05.043. Epub 2010 May 26. J Mol Biol. 2010. PMID: 20621650
-
Structural similarities of human and mammalian lipocalins, and their function in innate immunity and allergy.Allergy. 2016 Mar;71(3):286-94. doi: 10.1111/all.12797. Epub 2015 Nov 23. Allergy. 2016. PMID: 26497994 Free PMC article. Review.
-
An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen.Allergy Asthma Clin Immunol. 2018 Apr 10;14:14. doi: 10.1186/s13223-018-0239-8. eCollection 2018. Allergy Asthma Clin Immunol. 2018. PMID: 29643919 Free PMC article. Review.
Cited by
-
Role of Small Molecule Ligands in IgE-Mediated Allergy.Curr Allergy Asthma Rep. 2023 Sep;23(9):497-508. doi: 10.1007/s11882-023-01100-2. Epub 2023 Jun 23. Curr Allergy Asthma Rep. 2023. PMID: 37351723 Free PMC article. Review.
-
Malnutrition and Allergies: Tipping the Immune Balance towards Health.J Clin Med. 2024 Aug 11;13(16):4713. doi: 10.3390/jcm13164713. J Clin Med. 2024. PMID: 39200855 Free PMC article. Review.
-
Human IgE monoclonal antibodies define two unusual epitopes trapping dog allergen Can f 1 in different conformations.Protein Sci. 2025 Sep;34(9):e70269. doi: 10.1002/pro.70269. Protein Sci. 2025. PMID: 40828364 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

