Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial
- PMID: 36960162
- PMCID: PMC10028524
- DOI: 10.1111/irv.13116
Type I interferon autoantibodies in hospitalized patients with Middle East respiratory syndrome and association with outcomes and treatment effect of interferon beta-1b in MIRACLE clinical trial
Abstract
Background: Type I interferons (IFNs) are essential antiviral cytokines induced upon respiratory exposure to coronaviruses. Defects in type I IFN signaling can result in severe disease upon exposure to respiratory viral infection and are associated with worse clinical outcomes. Neutralizing autoantibodies (auto-Abs) to type I IFNs were reported as a risk factor for life-threatening COVID-19, but their presence has not been evaluated in patients with severe Middle East respiratory syndrome (MERS).
Methods: We evaluated the prevalence of type I IFN auto-Abs in a cohort of hospitalized patients with MERS who were enrolled in a placebo-controlled clinical trial for treatment with IFN-β1b and lopinavir-ritonavir (MIRACLE trial). Samples were tested for type I IFN auto-Abs using a multiplex particle-based assay.
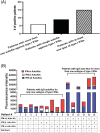
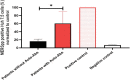
Results: Among the 62 enrolled patients, 15 (24.2%) were positive for immunoglobulin G auto-Abs for at least one subtype of type I IFNs. Auto-Abs positive patients were not different from auto-Abs negative patients in age, sex, or comorbidities. However, the majority (93.3%) of patients who were auto-Abs positive were critically ill and admitted to the ICU at the time of enrollment compared to 66% in the auto-Abs negative patients. The effect of treatment with IFN-β1b and lopinavir-ritonavir did not significantly differ between the two groups.
Conclusion: This study demonstrates the presence of type I IFN auto-Abs in hospitalized patients with MERS.
Keywords: ICU; MERS; MIRACLE trial; Type I IFNs; auto‐abs.
© 2023 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
YA provided nonpaid consultations on therapeutics for MERS for Gilead Sciences and SAB Biotherapeutics, and he is a board member of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). All other authors declare no financial or commercial conflict of interest.
Figures

References
-
- Organization WH . Middle East respiratory syndrome coronavirus (MERS‐CoV). 2023. doi: https://www.who.int/emergencies/mers-cov/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

