Prostate cancer bone metastases biology and clinical management (Review)
- PMID: 36960185
- PMCID: PMC10028493
- DOI: 10.3892/ol.2023.13749
Prostate cancer bone metastases biology and clinical management (Review)
Abstract
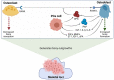
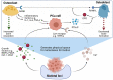
Prostate cancer (PCa) is one of the most prominent causes of cancer-related mortality in the male population. A highly impactful prognostic factor for patients diagnosed with PCa is the presence or absence of bone metastases. The formation of secondary tumours at the bone is the most commonly observed site for the establishment of PCa metastases and is associated with reduced survival of patients in addition to a cohort of life-debilitating symptoms, including mobility issues and chronic pain. Despite the prevalence of this disease presentation and the high medical relevance of bone metastases, the mechanisms underlying the formation of metastases to the bone and the understanding of what drives the osteotropism exhibited by prostate tumours remain to be fully elucidated. This lack of in-depth understanding manifests in limited effective treatment options for patients with advanced metastatic PCa and culminates in the low rate of survival observed for this sub-set of patients. The present review aims to summarise the most recent promising advances in the understanding of how and why prostate tumours metastasise to the bone, with the ultimate aim of highlighting novel treatment and prognostic targets, which may provide the opportunity to improve the diagnosis and treatment of patients with PCa with bone metastases.
Keywords: biomarker; bone metastases; osteoblasts; osteoclasts; prostate cancer; treatment.
Copyright: © Archer Goode et al.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
