Improvement influenza vaccine immune responses with traditional Chinese medicine and its active ingredients
- PMID: 36960292
- PMCID: PMC10027775
- DOI: 10.3389/fmicb.2023.1111886
Improvement influenza vaccine immune responses with traditional Chinese medicine and its active ingredients
Abstract
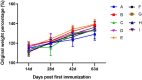
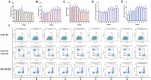
The current influenza vaccines are unable to provide effective protection in many cases, like influenza viruses strain antigenic drift or shift, and the influenza continues to cause significant annual morbidity and mortality. Improving the immune response to influenza vaccination is an unmet need. Traditional Chinese medicine (TCM) and its active ingredients are commonly known to have immunomodulatory properties. We therefore compared influenza vaccination alone or formulated with Astragali Radix (Huangqi in Chinese), and several representative ingredients of TCM, including lentinan (polysaccharide), panax notoginseng saponins (saponin), breviscapine (flavone), andrographolide (terpenoid), and a Chinese herbal compound (kangai) for their potential to enhance immune responses to influenza vaccine in mice. We found that all these TCM-adjuvants were able to increase hemagglutination inhibition (HAI) antibody titers, splenocyte proliferation, splenic T cell differentiation, bone marrow dendritic cell maturity, and both Th1 and Th2 cytokine secretion of influenza vaccine to varying degrees, and that had the characteristics of no excessive inflammatory responses and bidirectional regulation simultaneously. Taken together, our findings show that Astragali Radix exerts a more comprehensive effect on vaccine immunity, on both innate and adaptive immunity. The effects of lentinan and andrographolide on adaptive immunity were more significant, while the effects of breviscapine on innate immunity were stronger, and the other two TCM adjuvants were weaker. As the first report of a comprehensive evaluation of TCM adjuvants in influenza vaccines, the results suggest that TCM and their active ingredients are good candidates for enhancing the immune response of influenza vaccines, and that suitable TCMs can be selected based on the adjuvant requirements of different vaccines.
Keywords: active ingredients; adjuvant; immune responses; influenza vaccine; traditional Chinese medicine.
Copyright © 2023 Zhao, Chen, Wang, Zhang, Lv, Tan, Chen, Tao, Li, Chen, He and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials

