Effects of glioblastoma-derived extracellular vesicles on the functions of immune cells
- PMID: 36960410
- PMCID: PMC10028257
- DOI: 10.3389/fcell.2023.1060000
Effects of glioblastoma-derived extracellular vesicles on the functions of immune cells
Abstract
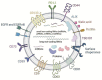
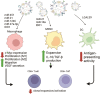
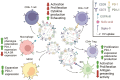
Glioblastoma is the most aggressive variant of glioma, the tumor of glial origin which accounts for 80% of brain tumors. Glioblastoma is characterized by astoundingly poor prognosis for patients; a combination of surgery, chemo- and radiotherapy used for clinical treatment of glioblastoma almost inevitably results in rapid relapse and development of more aggressive and therapy resistant tumor. Recently, it was demonstrated that extracellular vesicles produced by glioblastoma (GBM-EVs) during apoptotic cell death can bind to surrounding cells and change their phenotype to more aggressive. GBM-EVs participate also in establishment of immune suppressive microenvironment that protects glioblastoma from antigen-specific recognition and killing by T cells. In this review, we collected present data concerning characterization of GBM-EVs and study of their effects on different populations of the immune cells (T cells, macrophages, dendritic cells, myeloid-derived suppressor cells). We aimed at critical analysis of experimental evidence in order to conclude whether glioblastoma-derived extracellular vesicles are a major factor in immune evasion of this deadly tumor. We summarized data concerning potential use of GBM-EVs for non-invasive diagnostics of glioblastoma. Finally, the applicability of approaches aimed at blocking of GBM-EVs production or their fusion with target cells for treatment of glioblastoma was analyzed.
Keywords: T cell response; extracellular vesicles; glioblastoma multiforme; immune response; immune suppression; signaling pathways.
Copyright © 2023 Musatova and Rubtsov.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alix-Panabières C., Pantel K. (2021). Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 11, 858–873. 10.1158/2159-8290.CD-20-1311 - DOI - PubMed
-
- Antonyak M. A., Li B., Boroughs L. K., Johnson J. L., Druso J. E., Bryant K. L., et al. (2011). .Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA. 108, 4852–4857. 10.1073/pnas.1017667108 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

