Somatosensory Outcomes Following Re-Surgery in Persistent Severe Pain After Groin Hernia Repair: A Prospective Observational Study
- PMID: 36960467
- PMCID: PMC10030060
- DOI: 10.2147/JPR.S384973
Somatosensory Outcomes Following Re-Surgery in Persistent Severe Pain After Groin Hernia Repair: A Prospective Observational Study
Abstract
Purpose: After groin hernia repair (globally more than 20 million/year) 2-4% will develop persistent severe pain (PSPG). Pain management is challenging and may require multimodal interventions, including re-surgery. Quantitative somatosensory testing (QST) is an investigational psychophysiological tool with the potential to uncover the pathophysiological mechanisms behind the pain, ie, revealing neuropathic or inflammatory components. The primary objective was to examine and describe the underlying pathophysiological changes in the groin areas by QST before and after re-surgery with mesh removal and selective neurectomy.
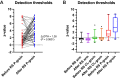
Patients and methods: Sixty patients with PSPG scheduled for re-surgery and with an inflammatory "component" indicated by blunt pressure algometry were examined in median (95% CI) 7.9 (5.8-11.5) months before and 4.0 (3.5-4.6) months after re-surgery. The QST-analyses included standardized assessments of cutaneous mechanical/thermal detection and pain thresholds. Suprathreshold heat stimuli were applied. Deep tissue sensitivity was tested by pressure algometry. Testing sites were the groin areas and the lower arm. Before/after QST data were z-transformed.
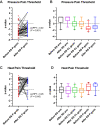
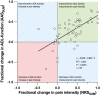
Results: Re-surgery resulted in median changes in rest, average, and maximal pain intensity scores of -2.0, -2.5, and -2.0 NRS (0/10) units, respectively (P = 0.0001), and proportional increases in various standardized functional scores (P = 0.0001). Compared with the control sites, the cutaneous somatosensory detection thresholds of the painful groin were increased before re-surgery and increased further after re-surgery (median difference: 1.28 z-values; P = 0.001), indicating a successive post-surgical loss of nerve fiber function ("deafferentation"). Pressure algometry thresholds increased after re-surgery (median difference: 0.30 z-values; P = 0.001).
Conclusion: In this subset of patients with PSPG who underwent re-surgery, the procedure was associated with improved pain and functional outcomes. While the increase in somatosensory detection thresholds mirrors the surgery-induced cutaneous deafferentation, the increase in pressure algometry thresholds mirrors the removal of the deep "pain generator". The QST-analyses are useful adjuncts in mechanism-based somatosensory research.
Keywords: chronic post-surgical pain; groin; hernia repair; reoperation; sensory thresholds.
© 2023 Jensen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2016;18(4):359.e351–359.e338. - PubMed
-
- Campanelli G, Bruni PG, Morlacchi A, Cavalli M. Chronic pain after inguinal hernia repair. In: Campanelli G, editor. Inguinal Hernia Surgery. Milano: Springer Milan; 2017:157–168.
LinkOut - more resources
Full Text Sources

