Drug-induced lung disease: a brief update for radiologists
- PMID: 36960496
- PMCID: PMC10679578
- DOI: 10.5152/dir.2022.21614
Drug-induced lung disease: a brief update for radiologists
Abstract
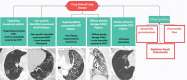
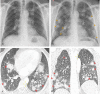
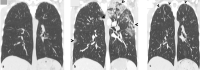
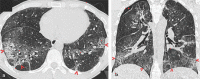
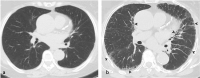
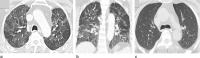
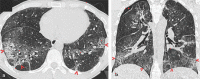
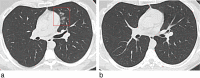
Pulmonary adverse events and drug-induced lung disease (DILD) can occur when treating many conditions. The incidence of DILDs in clinical practice and the variety of radiological findings have increased, mainly due to the increased use of novel therapeutic agents. It is crucial to determine whether the newly emerging clinical and imaging findings in these patients are due to the progression of the underlying disease, infection, pulmonary edema, or drug use, as this will change the patient management. Although the diagnosis of DILD is usually obtained by excluding other possible causes, radiologists should be aware of the imaging findings of DILD. This article reviews the essential radiological results of DILD and summarizes the critical clinical and imaging findings with an emphasis on novel therapeutic agents.
Keywords: Computed tomography; immunotherapy; lung; pneumonitis; pulmonary toxicity; therapy.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures



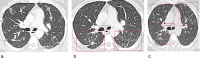
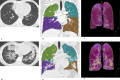
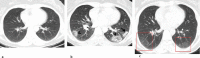
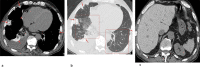
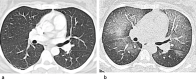
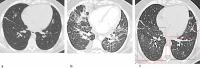
References
-
- Jessurun NT, Drent M, van Puijenbroek EP, Bekers O, Wijnen PA, Bast A. Druginduced interstitial lung disease: role of pharmacogenetics in predicting cytotoxic mechanisms and risks of side effects. Curr Opin Pulm Med. 2019;25(5):468–477. - PubMed
-
- Roden AC, Camus P. Iatrogenic pulmonary lesions. Semin Diagn Pathol. 2018;35(4):260–271. - PubMed
-
- Wang GX, Kurra V, Gainor JF, et al. Immune checkpoint inhibitor cancer therapy: spectrum of imaging findings. Radiographics. 2017;37(7):2132–2144. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

