STN7 is not essential for developmental acclimation of Arabidopsis to light intensity
- PMID: 36960687
- PMCID: PMC10952155
- DOI: 10.1111/tpj.16204
STN7 is not essential for developmental acclimation of Arabidopsis to light intensity
Abstract
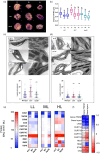
Plants respond to changing light intensity in the short term through regulation of light harvesting, electron transfer, and metabolism to mitigate redox stress. A sustained shift in light intensity leads to a long-term acclimation response (LTR). This involves adjustment in the stoichiometry of photosynthetic complexes through de novo synthesis and degradation of specific proteins associated with the thylakoid membrane. The light-harvesting complex II (LHCII) serine/threonine kinase STN7 plays a key role in short-term light harvesting regulation and was also suggested to be crucial to the LTR. Arabidopsis plants lacking STN7 (stn7) shifted to low light experience higher photosystem II (PSII) redox pressure than the wild type or those lacking the cognate phosphatase TAP38 (tap38), while the reverse is true at high light, where tap38 suffers more. In principle, the LTR should allow optimisation of the stoichiometry of photosynthetic complexes to mitigate these effects. We used quantitative label-free proteomics to assess how the relative abundance of photosynthetic proteins varied with growth light intensity in wild-type, stn7, and tap38 plants. All plants were able to adjust photosystem I, LHCII, cytochrome b6 f, and ATP synthase abundance with changing white light intensity, demonstrating neither STN7 nor TAP38 is crucial to the LTR per se. However, stn7 plants grown for several weeks at low light (LL) or moderate light (ML) still showed high PSII redox pressure and correspondingly lower PSII efficiency, CO2 assimilation, and leaf area compared to wild-type and tap38 plants, hence the LTR is unable to fully ameliorate these symptoms. In contrast, under high light growth conditions the mutants and wild type behaved similarly. These data are consistent with the paramount role of STN7-dependent LHCII phosphorylation in tuning PSII redox state for optimal growth in LL and ML conditions.
Keywords: Arabidopsis thaliana; acclimation; electron transfer; light harvesting; photosynthesis; photosystem; proteomics; signalling; thylakoid.
© 2023 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Adamiec, M. , Drath, M. & Jackowski, G. (2008) Redox state of plastoquinone pool regulates expression of Arabidopsis thaliana genes in response to elevated irradiance. Acta Biochimica Polonica, 55, 161–174. - PubMed
-
- Albertsson, P.A. , Andreasson, E. , Stefansson, H. & Wollenberger, L. (1994) Fractionation of thylakoid membrane. Methods in Enzymology, 228, 469–482.
-
- Anderson, J. , Chow, W. & Goodchild, D. (1988) Thylakoid membrane organisation in sun/shade acclimation. Australian Journal of Plant Physiology, 15, 11–26.
-
- Anderson, J.M. (1986) Photoregulation of the composition, function and structure of thylakoid membranes. Annual Review of Plant Physiology, 37, 93–136.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

