Tracking COVID-19 in the United States With Surveillance of Aggregate Cases and Deaths
- PMID: 36960828
- PMCID: PMC10040484
- DOI: 10.1177/00333549231163531
Tracking COVID-19 in the United States With Surveillance of Aggregate Cases and Deaths
Abstract
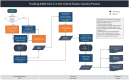
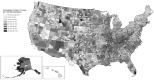
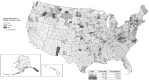
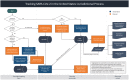
Early during the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) leveraged an existing surveillance system infrastructure to monitor COVID-19 cases and deaths in the United States. Given the time needed to report individual-level (also called line-level) COVID-19 case and death data containing detailed information from individual case reports, CDC designed and implemented a new aggregate case surveillance system to inform emergency response decisions more efficiently, with timelier indicators of emerging areas of concern. We describe the processes implemented by CDC to operationalize this novel, multifaceted aggregate surveillance system for collecting COVID-19 case and death data to track the spread and impact of the SARS-CoV-2 virus at national, state, and county levels. We also review the processes established to acquire, process, and validate the aggregate number of cases and deaths due to COVID-19 in the United States at the county and jurisdiction levels during the pandemic. These processes include time-saving tools and strategies implemented to collect and validate authoritative COVID-19 case and death data from jurisdictions, such as web scraping to automate data collection and algorithms to identify and correct data anomalies. This topical review highlights the need to prepare for future emergencies, such as novel disease outbreaks, by having an event-agnostic aggregate surveillance system infrastructure in place to supplement line-level case reporting for near-real-time situational awareness and timely data.
Keywords: CDC; COVID-19; data; public health; surveillance.
Conflict of interest statement
The findings and conclusions in this article are those of the authors and do not represent the official position of CDC.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Bush L, Malecki J, W, iersma S, et al.. Update: investigation of anthrax associated with intentional exposure and interim public health guidelines, October 2001. MMWR Morb Mortal Wkly Rep. 2001;50(41):889-893. - PubMed
-
- US Department of Defense. Review committee report: inadvertent shipment of live Bacillus anthracis spores by DoD. Committee for comprehensive review of DoD laboratory procedures, processes, and protocols associated with inactivating Bacillus anthracis spores. July13, 2015. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2015/0615_lab-stats/Review-Co...
-
- Fleischauer AT.Outbreak of severe acute respiratory syndrome—worldwide, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(11):226-228. - PubMed
-
- Fujita N, Miller A, Miller G, et al.. Imported case of Marburg hemorrhagic fever—Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(49):1377-1381. - PubMed
-
- Centers for Disease Control and Prevention. Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(17):467-470. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

