By what molecular mechanisms do social determinants impact cardiometabolic risk?
- PMID: 36960908
- PMCID: PMC10039705
- DOI: 10.1042/CS20220304
By what molecular mechanisms do social determinants impact cardiometabolic risk?
Abstract
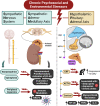
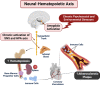
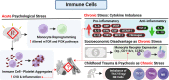
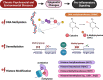
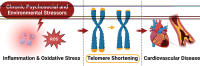
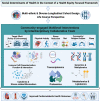
While it is well known from numerous epidemiologic investigations that social determinants (socioeconomic, environmental, and psychosocial factors exposed to over the life-course) can dramatically impact cardiovascular health, the molecular mechanisms by which social determinants lead to poor cardiometabolic outcomes are not well understood. This review comprehensively summarizes a variety of current topics surrounding the biological effects of adverse social determinants (i.e., the biology of adversity), linking translational and laboratory studies with epidemiologic findings. With a strong focus on the biological effects of chronic stress, we highlight an array of studies on molecular and immunological signaling in the context of social determinants of health (SDoH). The main topics covered include biomarkers of sympathetic nervous system and hypothalamic-pituitary-adrenal axis activation, and the role of inflammation in the biology of adversity focusing on glucocorticoid resistance and key inflammatory cytokines linked to psychosocial and environmental stressors (PSES). We then further discuss the effect of SDoH on immune cell distribution and characterization by subset, receptor expression, and function. Lastly, we describe epigenetic regulation of the chronic stress response and effects of SDoH on telomere length and aging. Ultimately, we highlight critical knowledge gaps for future research as we strive to develop more targeted interventions that account for SDoH to improve cardiometabolic health for at-risk, vulnerable populations.
Keywords: Cardiometabolic Disease; Chronic Stress; Immune System; Inflammation; Psychosocial and Environmental Stressors; Social Determinants of Health.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

