Supporting Biomarker-Driven Therapies in Oncology: A Genomic Testing Cost Calculator
- PMID: 36961477
- PMCID: PMC10166172
- DOI: 10.1093/oncolo/oyad005
Supporting Biomarker-Driven Therapies in Oncology: A Genomic Testing Cost Calculator
Abstract
Background: Adoption of high-throughput, gene panel-based, next-generation sequencing (NGS) into routine cancer care is widely supported, but hampered by concerns about cost. To inform policies regarding genomic testing strategies, we propose a simple metric, cost per correctly identified patient (CCIP), that compares sequential single-gene testing (SGT) vs. multiplex NGS in different tumor types.
Materials and methods: A genomic testing cost calculator was developed based on clinically actionable genomic alterations identified in the European Society for Medical Oncology Scale for Clinical Actionability of molecular Targets. Using sensitivity/specificity data for SGTs (immunohistochemistry, polymerase chain reaction, and fluorescence in situ hybridization) and NGS and marker prevalence, the number needed to predict metric was monetarized to estimate CCIP.
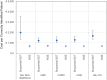
Results: At base case, CCIP was lower with NGS than sequential SGT for advanced/metastatic non-squamous non-small cell lung cancer (NSCLC), breast, colorectal, gastric cancers, and cholangiocarcinoma. CCIP with NGS was also favorable for squamous NSCLC, pancreatic, and hepatic cancers, but with overlapping confidence intervals. CCIP favored SGT for prostate cancer. Alternate scenarios using different price estimates for each test showed similar trends, but with incremental changes in the magnitude of difference between NGS and SGT, depending on price estimates for each test.
Conclusions: The cost to correctly identify clinically actionable genomic alterations was lower for NGS than sequential SGT in most cancer types evaluated. Decreasing price estimates for NGS and the rapid expansion of targeted therapies and accompanying biomarkers are anticipated to further support NGS as a preferred diagnostic standard for precision oncology.
Keywords: biomarker; calculator; next-generation sequencing; precision oncology.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Albrecht Stenzinger: member of the advisory board/speaker’s bureau for Aignostics, Amgen, AstraZeneca, Bayer, BMS, Eli Lilly, Illumina, Incyte, Janssen, MSD, Novartis, Pfizer, Qlucore, Roche, Seattle Genetics, Takeda, and Thermo Fisher Scientific, and receiving grants from Bayer, BMS, Chugai, and Incyte. Brian Cuffel and Noman Paracha: employees and stockholders of Bayer. Eric Vail: employee and stockholder of LungLife AI, receives honoraria from Thermo Fisher Scientific and Illumina, serves in a consulting/advisory role for Tempus Labs, Inc. and PierianDx, and is a member of the speakers’ bureau for Bayer, Eli Lilly, AstraZeneca, and Janssen Oncology. Jesus Garcia-Foncillas: reported consulting/advisory, honoraria, and speaker roles with Abbott, Amgen, Astellas, AstraZeneca, Biocartis, Boehringer Ingelheim, BMS, Bayer, Celgene, Eisai, Foundation Medicine, GSK, Hospira, Janssen, Lilly, Merck Serono, MSD, Novartis, Pharmamar, Pfizer, Roche, Sanofi, Servier, Sysmex, and Tesaro. Clifford Goodman: employee of The Lewin Group, Inc., which is a business unit of Optum, a wholly owned subsidiary of UnitedHealth Group, is a stockholder of UnitedHealth Group, and has consulting/advisory roles as part of employment for The Lewin Group for Bayer, BioMarin, Concert Genetics, Magellan Health, Medtronic, Merck, Sandoz, and Roche. Ulrik Lassen: received research grants from BMS, GSK, Pfizer, and Roche, and serves on the advisory boards for Bayer, Novartis, and Pfizer. Gilles Vassal: advisor to Bayer, BMS, Roche/Genentech, Celgene, Debiopharm, Incyte, Ipsen, Lilly, Pfizer, Servier, and Takeda, without personal remuneration. Sean D. Sullivan: serves on the advisory board for Bayer, Incyte, Novartis Gene Therapy, and Neurocrine, and has received grants from Bayer, Incyte, Novo Nordisk, and Neurocrine.
Figures

References
-
- Yoshino T, Pentheroudakis G, Mishima S, et al. . JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol. 2020;31(7):861-872. 10.1016/j.annonc.2020.03.299 - DOI - PubMed
-
- Lavacchi D, Roviello G, D’Angelo A.. Tumor-agnostic treatment for cancer: when how is better than where. Clin Drug Invest. 2020;40(6):519-527. - PubMed
-
- Schwegler C, Kaufmann D, Pfeiffer D, et al. . Population-level effect of molecular testing and targeted therapy in patients with advanced pulmonary adenocarcinoma: a prospective cohort study. Virchows Arch. 2018;472(4):581-588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

