Functional and molecular determinants of right ventricular response to severe pulmonary hypertension in a large animal model
- PMID: 36961489
- PMCID: PMC10190846
- DOI: 10.1152/ajpheart.00614.2022
Functional and molecular determinants of right ventricular response to severe pulmonary hypertension in a large animal model
Abstract
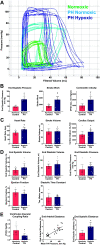
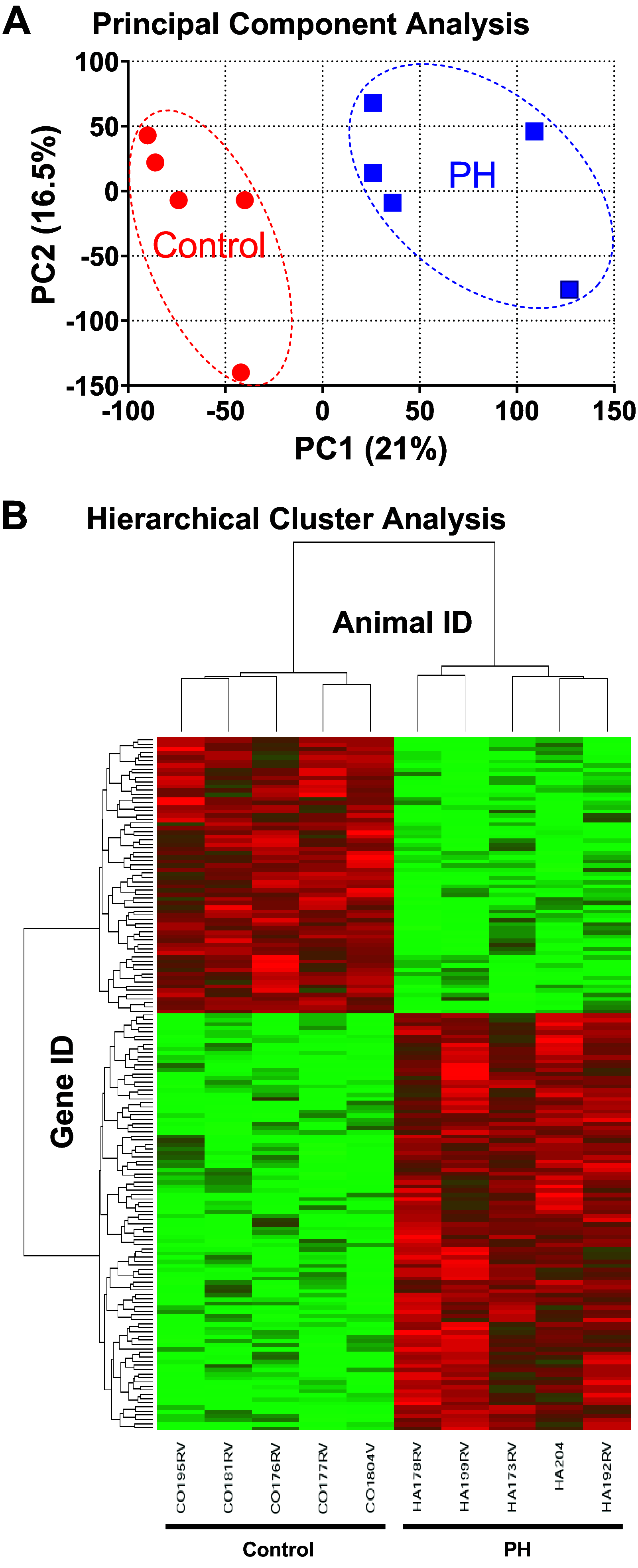
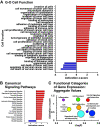
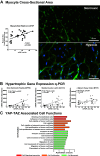
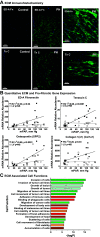
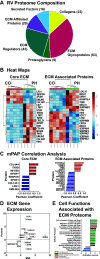
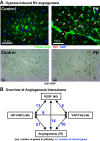
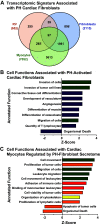
Right ventricular (RV) failure is the major determinant of outcome in pulmonary hypertension (PH). Calves exposed to 2-wk hypoxia develop severe PH and unlike rodents, hypoxia-induced PH in this species can lead to right heart failure. We, therefore, sought to examine the molecular and structural changes in the RV in calves with hypoxia-induced PH, hypothesizing that we could identify mechanisms underlying compensated physiological function in the face of developing severe PH. Calves were exposed to 14 days of environmental hypoxia (equivalent to 4,570 m/15,000 ft elevation, n = 29) or ambient normoxia (1,525 m/5,000 ft, n = 25). Cardiopulmonary function was evaluated by right heart catheterization and pressure volume loops. Molecular and cellular determinants of RV remodeling were analyzed by cDNA microarrays, RealTime PCR, proteomics, and immunochemistry. Hypoxic exposure induced robust PH, with increased RV contractile performance and preserved cardiac output, yet evidence of dysregulated RV-pulmonary artery mechanical coupling as seen in advanced disease. Analysis of gene expression revealed cellular processes associated with structural remodeling, cell signaling, and survival. We further identified specific clusters of gene expression associated with 1) hypertrophic gene expression and prosurvival mechanotransduction through YAP-TAZ signaling, 2) extracellular matrix (ECM) remodeling, 3) inflammatory cell activation, and 4) angiogenesis. A potential transcriptomic signature of cardiac fibroblasts in RV remodeling was detected, enriched in functions related to cell movement, tissue differentiation, and angiogenesis. Proteomic and immunohistochemical analysis confirmed RV myocyte hypertrophy, together with localization of ECM remodeling, inflammatory cell activation, and endothelial cell proliferation within the RV interstitium. In conclusion, hypoxia and hemodynamic load initiate coordinated processes of protective and compensatory RV remodeling to withstand the progression of PH.NEW & NOTEWORTHY Using a large animal model and employing a comprehensive approach integrating hemodynamic, transcriptomic, proteomic, and immunohistochemical analyses, we examined the early (2 wk) effects of severe PH on the RV. We observed that RV remodeling during PH progression represents a continuum of transcriptionally driven processes whereby cardiac myocytes, fibroblasts, endothelial cells, and proremodeling macrophages act to coordinately maintain physiological homeostasis and protect myocyte survival during chronic, severe, and progressive pressure overload.
Keywords: adaptation; pulmonary hypertension; right ventricle.
Conflict of interest statement
G.M.K. is employed by Innotiv/Bolder Biopath, Boulder, CO. M.G.E. is owner of Bioinfo Solutions, LLC, Parker, CO. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


Similar articles
-
Right Ventricular Sarcomere Contractile Depression and the Role of Thick Filament Activation in Human Heart Failure With Pulmonary Hypertension.Circulation. 2023 Jun 20;147(25):1919-1932. doi: 10.1161/CIRCULATIONAHA.123.064717. Epub 2023 May 17. Circulation. 2023. PMID: 37194598 Free PMC article.
-
Right Ventricular Stiffening and Anisotropy Alterations in Pulmonary Hypertension: Mechanisms and Relations to Right Heart Failure.J Am Heart Assoc. 2025 Mar 4;14(5):e037126. doi: 10.1161/JAHA.124.037126. Epub 2025 Feb 26. J Am Heart Assoc. 2025. PMID: 40008537 Free PMC article.
-
3D Imaging Reveals Complex Microvascular Remodeling in the Right Ventricle in Pulmonary Hypertension.Circ Res. 2024 Jun 21;135(1):60-75. doi: 10.1161/CIRCRESAHA.123.323546. Epub 2024 May 21. Circ Res. 2024. PMID: 38770652 Free PMC article.
-
Current landscape for connective tissue disease associated-pulmonary arterial hypertension: a focus on right ventricular adaptation and failure.Future Cardiol. 2025 Aug;21(10):803-814. doi: 10.1080/14796678.2025.2529696. Epub 2025 Jul 11. Future Cardiol. 2025. PMID: 40641351 Review.
-
Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review.Crit Care. 2010;14(5):R169. doi: 10.1186/cc9264. Epub 2010 Sep 21. Crit Care. 2010. PMID: 20858239 Free PMC article.
Cited by
-
Characterizing the Spatiotemporal Transcriptomic Response of the Right Ventricle to Acute Pressure Overload.Int J Mol Sci. 2023 Jun 5;24(11):9746. doi: 10.3390/ijms24119746. Int J Mol Sci. 2023. PMID: 37298696 Free PMC article.
-
Cardiac fibroblasts: answering the call.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H681-H686. doi: 10.1152/ajpheart.00478.2024. Epub 2024 Aug 2. Am J Physiol Heart Circ Physiol. 2024. PMID: 39093000 Free PMC article. Review.
-
Precision Medicine for Pulmonary Vascular Disease: The Future Is Now (2023 Grover Conference Series).Pulm Circ. 2025 Jan 2;15(1):e70027. doi: 10.1002/pul2.70027. eCollection 2025 Jan. Pulm Circ. 2025. PMID: 39749110 Free PMC article.
-
Echocardiographic Changes Related to Pulmonary Hypertension in Preweaned Dairy Calves With Bronchopneumonia: A Case-Control Study in Commercial Dairy Farms.J Vet Intern Med. 2025 Mar-Apr;39(2):e70020. doi: 10.1111/jvim.70020. J Vet Intern Med. 2025. PMID: 39957534 Free PMC article.
-
Pathophysiology of the right ventricle and its pulmonary vascular interaction.Eur Respir J. 2024 Oct 31;64(4):2401321. doi: 10.1183/13993003.01321-2024. Print 2024 Oct. Eur Respir J. 2024. PMID: 39209482 Free PMC article. Review.
References
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, , et al. . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009. doi:10.1161/CIRCULATIONAHA.109.192230. - DOI - PubMed
-
- Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, Kawut SM, Langleben D, Lumens J, Naeije R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 53: 1801900, 2019. doi:10.1183/13993003.01900-2018. - DOI - PMC - PubMed
-
- Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62: D22–D33, 2013. doi:10.1016/j.jacc.2013.10.027. - DOI - PubMed
-
- Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB; National Heart L, Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006. doi:10.1161/CIRCULATIONAHA.106.632208. - DOI - PubMed
-
- Cornwell WK, Tran T, Cerbin L, Coe G, Muralidhar A, Hunter K, Altman N, Ambardekar AV, Tompkins C, Zipse M, Schulte M, O'Gean K, Ostertag M, Hoffman J, Pal JD, Lawley JS, Levine BD, Wolfel E, Kohrt WM, Buttrick P. New insights into resting and exertional right ventricular performance in the healthy heart through real-time pressure-volume analysis. J Physiol 598: 2575–2587, 2020. doi:10.1113/JP279759. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

