Evidence for virus-mediated oncogenesis in bladder cancers arising in solid organ transplant recipients
- PMID: 36961501
- PMCID: PMC10446826
- DOI: 10.7554/eLife.82690
Evidence for virus-mediated oncogenesis in bladder cancers arising in solid organ transplant recipients
Abstract
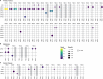
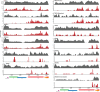
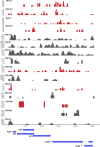
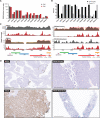
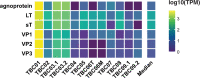
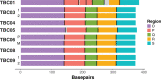
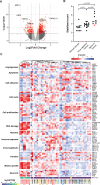
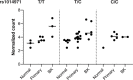
A small percentage of bladder cancers in the general population have been found to harbor DNA viruses. In contrast, up to 25% of tumors of solid organ transplant recipients, who are at an increased risk of developing bladder cancer and have an overall poorer outcomes, harbor BK polyomavirus (BKPyV). To better understand the biology of the tumors and the mechanisms of carcinogenesis from potential oncoviruses, we performed whole genome and transcriptome sequencing on bladder cancer specimens from 43 transplant patients. Nearly half of the tumors from this patient population contained viral sequences. The most common were from BKPyV (N=9, 21%), JC polyomavirus (N=7, 16%), carcinogenic human papillomaviruses (N=3, 7%), and torque teno viruses (N=5, 12%). Immunohistochemistry revealed variable Large T antigen expression in BKPyV-positive tumors ranging from 100% positive staining of tumor tissue to less than 1%. In most cases of BKPyV-positive tumors, the viral genome appeared to be clonally integrated into the host chromosome consistent with microhomology-mediated end joining and coincided with focal amplifications of the tumor genome similar to other virus-mediated cancers. Significant changes in host gene expression consistent with the functions of BKPyV Large T antigen were also observed in these tumors. Lastly, we identified four mutation signatures in our cases, with those attributable to APOBEC3 and SBS5 being the most abundant. Mutation signatures associated with an antiviral drug, ganciclovir, and aristolochic acid, a nephrotoxic compound found in some herbal medicines, were also observed. The results suggest multiple pathways to carcinogenesis in solid organ transplant recipients with a large fraction being virus-associated.
Keywords: bladder cancer; cancer biology; human; infectious disease; microbiology; papillomavirus; polyomavirus; solid organ transplant recipient; torque teno virus.
Conflict of interest statement
GS, KY, MP, DP, MD, AI, BH, TT, IC, LG, CM, SH, CL, LP, PM, CB, EE No competing interests declared, YG, PL is affiliated with Leidos Biomedical Research Inc. The author has no financial interests to declare, RH is a co-founder, shareholder, and consultant of ApoGen Biotechnologies Inc
Figures






Update of
References
-
- Ahasan MM, Wakae K, Wang Z, Kitamura K, Liu G, Koura M, Imayasu M, Sakamoto N, Hanaoka K, Nakamura M, Kyo S, Kondo S, Fujiwara H, Yoshizaki T, Mori S, Kukimoto I, Muramatsu M. Apobec3A and 3C decrease human papillomavirus 16 pseudovirion infectivity. Biochemical and Biophysical Research Communications. 2015;457:295–299. doi: 10.1016/j.bbrc.2014.12.103. - DOI - PubMed
-
- Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, Jiang B, Rocco JW, Teknos TN, Kumar B, Wangsa D, He D, Ried T, Symer DE, Gillison ML. Genome-Wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Research. 2014;24:185–199. doi: 10.1101/gr.164806.113. - DOI - PMC - PubMed
-
- Alexiev BA, Randhawa P, Vazquez Martul E, Zeng G, Luo C, Ramos E, Drachenberg CB, Papadimitriou JC. Bk virus-associated urinary bladder carcinoma in transplant recipients: report of 2 cases, review of the literature, and proposed pathogenetic model. Human Pathology. 2013;44:908–917. doi: 10.1016/j.humpath.2012.09.019. - DOI - PubMed
-
- Baker SC, Mason AS, Slip RG, Skinner KT, Macdonald A, Masood O, Harris RS, Fenton TR, Periyasamy M, Ali S, Southgate J. Induction of APOBEC3-mediated genomic damage in urothelium implicates BK polyomavirus (bkpyv) as a Hit-and-run driver for bladder cancer. Oncogene. 2022;41:2139–2151. doi: 10.1038/s41388-022-02235-8. - DOI - PMC - PubMed
-
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. - DOI - PMC - PubMed

