Comparative Efficacy of Teclistamab Versus Current Treatments in Real-World Clinical Practice in the Prospective LocoMMotion Study in Patients with Triple-Class-Exposed Relapsed and/or Refractory Multiple Myeloma
- PMID: 36961654
- PMCID: PMC10129954
- DOI: 10.1007/s12325-023-02480-7
Comparative Efficacy of Teclistamab Versus Current Treatments in Real-World Clinical Practice in the Prospective LocoMMotion Study in Patients with Triple-Class-Exposed Relapsed and/or Refractory Multiple Myeloma
Abstract
Introduction: Patients with triple-class-exposed relapsed/refractory multiple myeloma (TCE-RRMM) have a poor prognosis and limited treatment options. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, was studied in patients with TCE-RRMM in the single-arm MajesTEC-1 study. To assess the relative effectiveness of teclistamab versus real-world physician's choice of therapy (RWPC), adjusted comparisons were performed using individual patient data from MajesTEC-1 and LocoMMotion, a prospective study of patients with TCE-RRMM.
Methods: An external control arm for MajesTEC-1 was created from patients in LocoMMotion (n = 248; clinical cut-off: November 2, 2021) and compared with treated patients (n = 165) from MajesTEC-1 (teclistamab 1.5 mg/kg weekly; clinical cut-off: March 16, 2022). Inverse probability weighting was used to adjust for imbalances in baseline covariates. For binary endpoints [overall response rate (ORR), very good partial response or better (≥ VGPR) rate, complete response or better (≥ CR)], relative effect of teclistamab versus RWPC was estimated with an odds ratio and relative response rate and 95% confidence interval (CI), derived from weighted logistic regression. Weighted Cox proportional hazards model was used to estimate hazard ratios (HR) and 95% CIs for time-to-event endpoints [duration of response (DOR), progression-free survival (PFS), and overall survival (OS)].
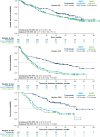
Results: After weighting, baseline characteristics were balanced across cohorts. In adjusted comparisons, teclistamab-treated patients were 2.3-fold, 5.2-fold and 148.3-fold, more likely to reach ORR [response-rate ratio (RR) = 2.31, 95% CI 1.77-2.85, p < 0.0001], ≥ VGPR (RR = 5.19, 95% CI 3.26-7.12, p < 0.0001) and ≥ CR (RR = 148.25, 95% CI 20.63-1065.40, p < 0.0001), respectively, versus patients receiving RWPC. Following adjustment, DOR (HR 0.32, 95% CI 0.19-0.54, p < 0.0001) and PFS (HR 0.48, 95% CI 0.35-0.65, p < 0.0001) were significantly longer with teclistamab versus RWPC. OS was numerically better with teclistamab versus RWPC [HR 0.77 (0.55-1.09), p = 0.1419].
Conclusion: Teclistamab demonstrated improved effectiveness versus RWPC, highlighting its clinical benefit as a novel and effective treatment for patients with TCE-RRMM.
Trial registration: Majest TEC-1, ClinicalTrials.gov NCT04557098; LocoMMotion, ClinicalTrials.gov NCT04035226.
Keywords: LocoMMotion; MajesTEC-1; Teclistamab; Triple-class exposed relapsed or refractory multiple myeloma.
© 2023. The Author(s).
Conflict of interest statement
Philippe Moreau has held a consulting or advisory role for AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Oncopeptides, and Sanofi; and received honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Oncopeptides, and Sanofi. Niels W. C. J. van de Donk has received honoraria from Celgene; held a consulting or advisory role for Amgen, Bayer, Celgene, Janssen, Novartis, Roche, Servier, and Takeda; and received research funding from Amgen, BMS, Cellectis, and Janssen. Michel Delforge has held a consulting or advisory role for Amgen, Celgene, and Janssen; and received honoraria from Amgen, Celgene, and Janssen. Hermann Einsele has held a consulting or advisory role for Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, and Takeda; received travel, accommodations, and/or expenses from Amgen, Bristol Myers Squibb, Celgene, Janssen, and Takeda; received honoraria from Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, and Takeda; and received research funding from Amgen, Bristol Myers Squibb, Celgene, and Janssen. Valerio De Stefano has received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi, and Takeda. Aurore Perrot has received honoraria from AbbVie, Amgen, Celgene, Janssen-Cilag, GlaxoSmithKline, Sanofi, and Takeda. Britta Besemer has received travel, accommodations, and/or expenses from Janssen-Cilag; and received honoraria from Janssen-Cilag. Charlotte Pawlyn has received honoraria from Amgen, Celgene/Bristol Myers Squibb, Janssen, Sanofi, and Takeda. Lionel Karlin reports employment (family member) by Aguettant; has received honoraria from AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Takeda; has held a consulting or advisory role for AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Takeda; and has received travel, accommodations, and/or expenses from Amgen, Janssen, Sanofi, and Takeda. Salomon Manier has received research funding from AbbVie, Celgene/Bristol Myers Squibb, Janssen, Novartis, and Takeda. Xavier Leleu has held a consulting or advisory role for AbbVie, Amgen, Bristol Myers Squibb, CARsgen Therapeutics, Celgene, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Merck, Novartis, Oncopeptides, Roche, and Takeda; received travel, accommodations, and/or expenses from Takeda; and received honoraria from AbbVie, Amgen, Bristol Myers Squibb, CARsgen Therapeutics, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Merck, Novartis, Oncopeptides, Roche, Sanofi, and Takeda. Katja Weisel has held a consulting or advisory role for Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Oncopeptides, Roche, Sanofi, and Takeda; has received travel, accommodations, and/or expenses from Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, and Takeda; received honoraria from AbbVie, Adaptive Biotechnologies, Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Novartis, Oncopeptides, Pfizer, Roche/Genentech, Sanofi, and Takeda; and has received research funding from Amgen, Bristol Myers Squibb/Celgene, Celgene, GlaxoSmithKline, Janssen-Cilag, and Sanofi. Francesca Ghilotti is employed by Janssen. Joris Diels is employed by and has stock/other ownership interests in Janssen. Ahmed Elsada was employed by Janssen-Cilag during the conduct of the research and publication development; he is currently an employee of Bristol-Myers Squibb. Raul Morano is employed by Janssen. Vadim Strulev is employed by and has stock/other ownership interests in Janssen. Lixia Pei is employed by and has stock/other ownership interests in Janssen. Rachel Kobos is employed by and has stock/other ownership interests in Janssen. Jennifer Smit is employed by, has stock/other ownership interests in, and has received travel, accommodations, and/or expenses from Johnson & Johnson/Janssen. Mary Slavcev is employed by and has stock/other ownership interests in Janssen. Maria-Victoria Mateos has held a consulting or advisory role for AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Regeneron, Roche/Genentech, and Takeda; and received honoraria from AbbVie/Genentech, Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi, and Takeda.
Figures

References
-
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

